NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 11, “Alcohols, Phenols, and Ethers,” is a study material package designed to help students understand the properties, preparation, and uses of alcohols, phenols, and ethers.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including the classification, nomenclature, and structure of alcohols, phenols, and ethers. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the physical properties of alcohols and phenols, such as their boiling points and solubility, to the chemical reactions of ethers and their uses as solvents. It also includes questions on the preparation of alcohols and phenols, including the various methods used for their synthesis.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the classification of alcohols, phenols, and ethers, their physical and chemical properties, and the different methods used for their preparation.
NCERT Solution / Notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers with Mind Map PDF Download
Alcohols, Phenols and Ethers
- Alcohols and phenols are compounds formed when a hydrogen atom in a hydrocarbon is replaced by–OH group.
- An alcohol contains one or more hydroxyl (OH) group(s) directly attached to carbon atom(s) of an aliphatic system.
- A phenol contains –OH group(s) directly attached to carbon atom(s) of an aromatic system (C6H5OH).
- The substitution of a hydrogen atom in a hydrocarbon by an alkoxy or aryloxy group (R-O/Ar-O) gives another class of compounds known as ethers.
For example: C2H5-O-C2H5 (Dimethyl ether)
Nomenclature
Alcohols
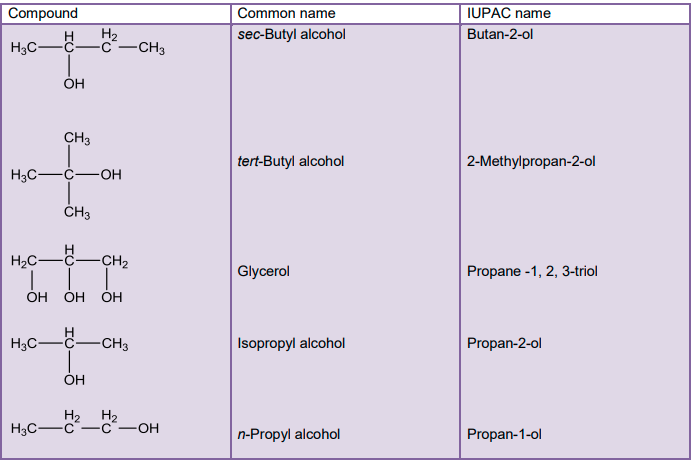
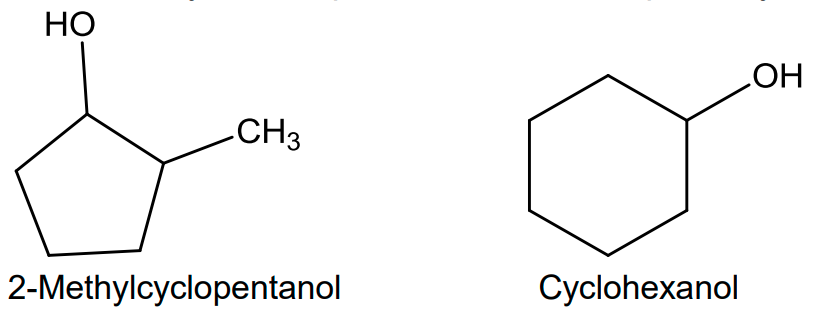
In case of cyclic compounds, we use the prefix cyclo if the –OH group is attached to C-1.
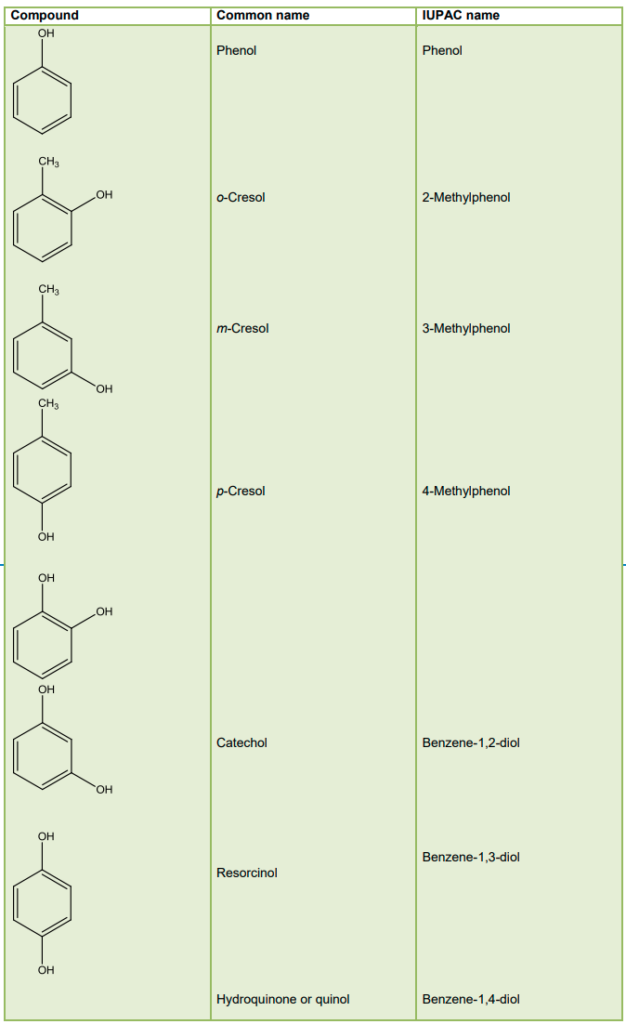
Phenols:
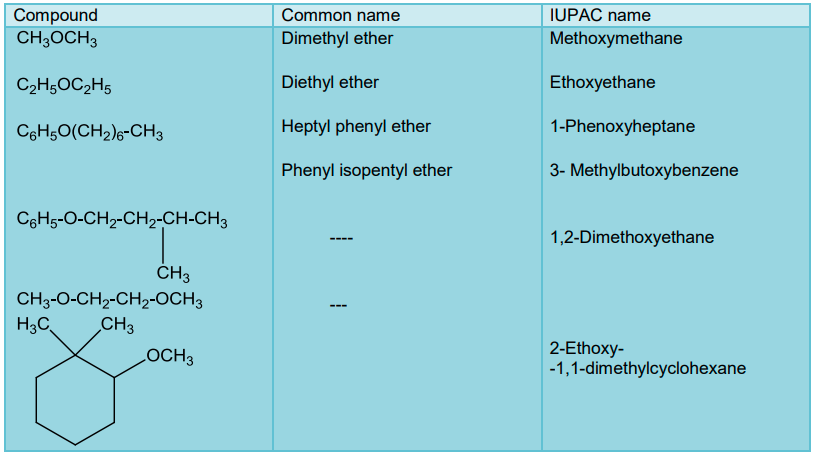
Ethers
Structures of Functional Groups
Alcohols
- For alcohols, the –OH group is linked to carbon by a sigma bond.
- The bond is formed by the overlap of sp3 hybridised orbital of carbon with a sp3 hybridised orbital of oxygen.
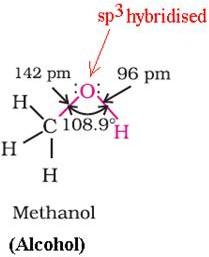
- In alcohols, the bond angle is slightly less than the tetrahedral angle (109°-28’) due to the repulsion between the unshared electron pairs of oxygen.
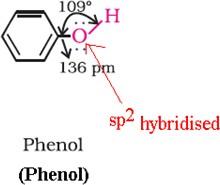
Phenols
- In phenols, the –OH group is linked to carbon by sp2 hybridisation.
- The C-O bond length (136 pm) in phenol is slightly less than that in methanol.
- This arises due to:
- Partial double bond character on account of the conjugation of unshared electron pair of oxygen with the aromatic ring.
- sp2 hybridised carbon to which oxygen is linked.
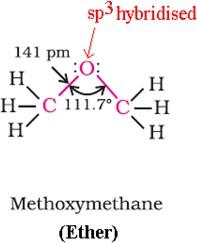
Ethers
- In ethers the two bond pairs and two lone pairs of electrons on oxygen form a tetrahedral arrangement.
- Due to the repulsive interaction between the two bulky (-R) groups the bond angle is slightly greater than the tetrahedral angle.
- The C-O bond length is almost the same like alcohols.
Preparation of Alcohols
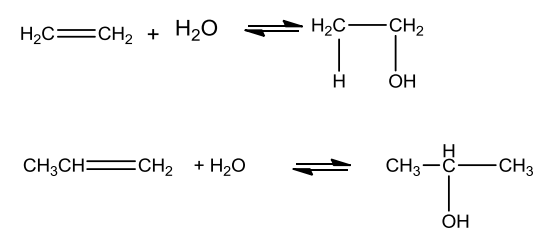
From Alkenes
- Acid catalysed hydration:
Alcohols are prepared by treating alkenes with water in the presence of acid as catalyst.
- Hydroboration-oxidation:
Alkenes on treatment with diborane give trialkyl boranes as addition product which is then oxidised to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.
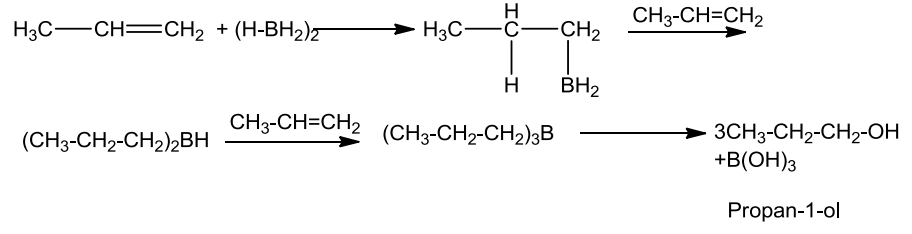
The addition of borane to the double bond takes place in such a way that the boron gets added to the sp2 carbon with more number of hydrogen atoms.
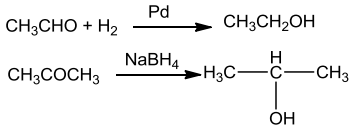
From Carbonyl Compounds
Reduction of Aldehydes & Ketones
- Aldehydes yield primary alcohols whereas ketones give secondary alcohols.
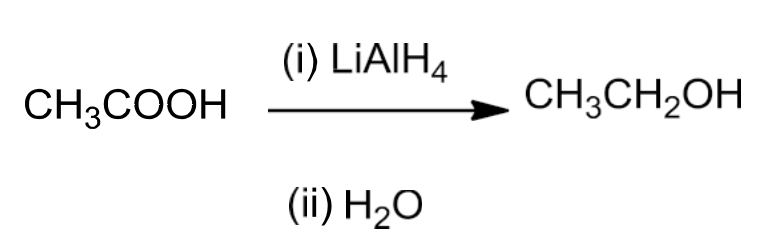
Reduction of Carboxylic acids and Esters
- LiAlH4 is a strong reducing agent and reduces carboxylic acids to primary alcohols in excellent yields.
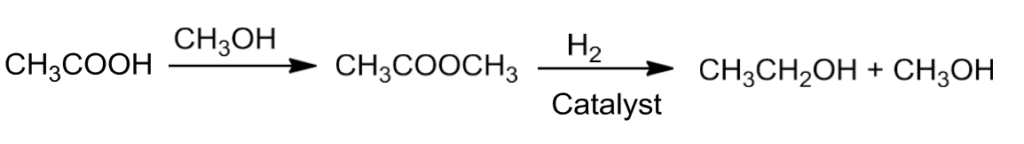
- Alcohols are prepared on a commercial scale by converting acids to esters followed by reduction with hydrogen in the presence of catalyst.
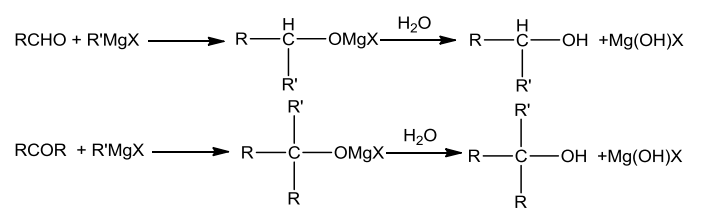
From Grignard reagents
Grignard reagents on reacting with aldehydes and ketones yield alcohols.
Preparation of Phenols
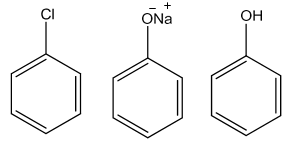
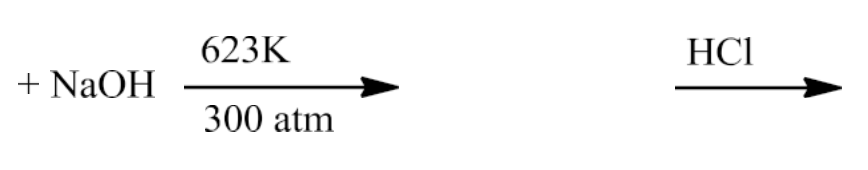
- From Haloarenes
Chlorobenzene on fusing with NaOH at 623 K and 320 atmospheric pressure gives sodium phenoxide which on acidification yields phenol.
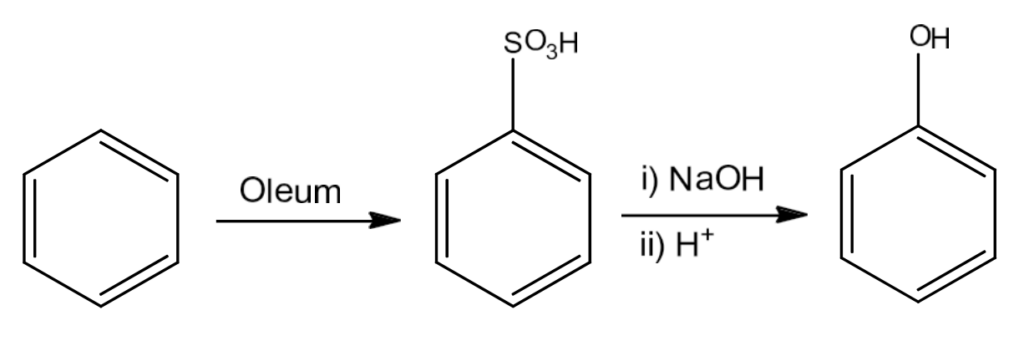
- From Benzenesulphonic Acid
Benzene on sulphonation with oleum gives benzene sulphonic acid which on heating with molten sodium hydroxide gives sodium phenoxide. Acidification of the sodium phenoxide gives phenol.
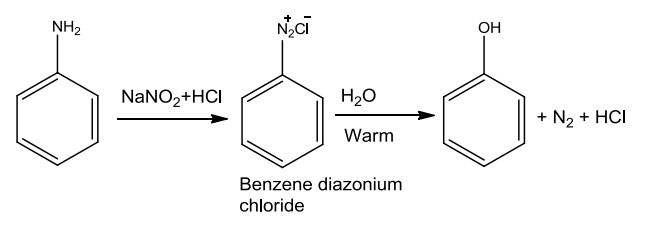
- From Diazonium Salts
Aniline on treatment with nitrous acid at 273-278K gives benzene diazonium chloride which on hydrolysis with warm water or treatment with dilute acids is converted to phenols.
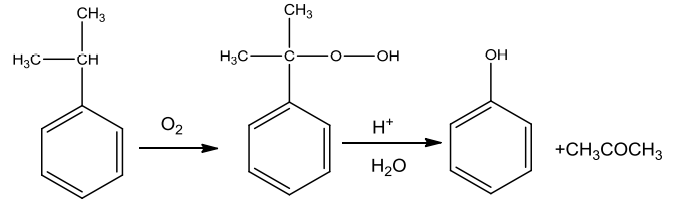
- From Cumene
Cumene(isopropylbenzene) on oxidation with air gives cumene hydroperoxide which on treatment with dilute acid is converted to phenol.
Physical Properties
- Boiling points
- Boiling points of alcohols and phenols are higher in comparison to other classes of compounds, namely hydrocarbons, ethers, haloalkanes and haloarenes of comparable molecular masses. This is because the –OH group in alcohols and phenols is involved in intermolecular hydrogen bonding.
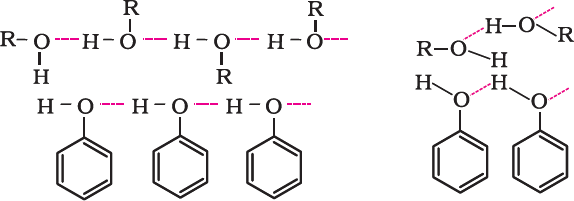
- The boiling points of alcohols and phenols increase with increase in the number of carbon atoms. This is because of increase in van der Waals forces with increase in the surface area.
- In alcohols, the boiling points decrease with increase in branching in the carbon chain. This is because of decrease in van der Waals forces with decrease in the surface area.
- Solubility
- Alcohols and phenols are soluble in water due to their ability to form hydrogen bonds with water molecules.
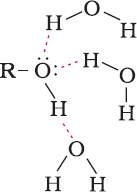
- The solubility of alcohols decreases with increase in the size of alkyl/aryl (hydrophobic) groups.
Chemical Properties
Alcohols react both as nucleophiles and electrophiles.
- Reactions involving cleavage of O-H bond
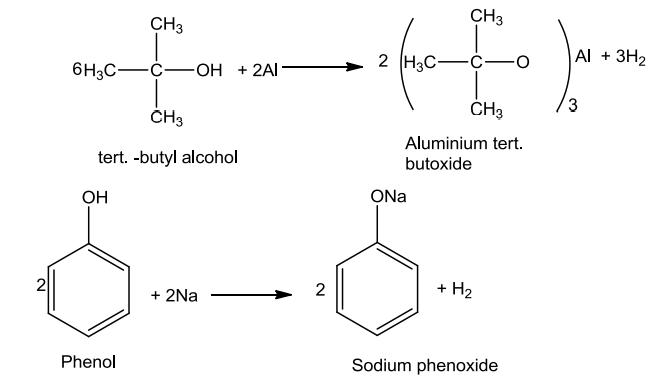
- Reaction with Metals
Alcohols and phenols react with active metals like Na, K and Al to give corresponding alkoxides/phenoxides with the evolution of hydrogen.
2R-OH + 2Na → 2R-O-Na + H2
- Acidity of Alcohols
- The acidity of alcohols depends on the polar nature of O-H bond.
- The electron releasing groups (-CH3, -C2H5) increases the electron density on oxygen and thus decrease the polarity of O-H bond which decreases the acid strength.
- The acid strength of alcohols decreases in the following order:
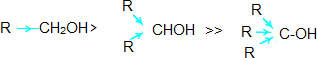
- Alcohols are weaker acids than water which can be seen in the following reaction.
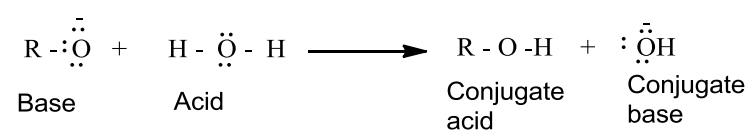
In the reaction, water is a better proton donor (i.e., stronger acid) than alcohol. Over here the alkoxide ion is a better proton acceptor than hydroxide ion which suggests that alkoxides are stronger bases.
- Alcohols act as Bronsted bases as well due to the presence of unshared electron pairs on oxygen which makes them proton acceptors.
- Acidity of Phenols
- In phenol, the hydroxyl group is directly attached to the sp2 hybridised carbon of the benzene ring which acts as an electron-withdrawing group. Whereas in alcohols, the hydroxyl group is attached to the alkyl group which has an electron-releasing inductive effect.
- In phenol, the hydroxyl group is directly attached to the sp2 hybridised carbon of the benzene ring. Whereas in alcohols, the hydroxyl group is attached to the sp3 hybridised carbon of the alkyl group. The sp2 hybridised carbon has higher electronegativity than the sp3 hybridised carbon. Thus, the polarity of the O–H bond of phenols is higher than that of alcohols. Hence, the ionisation of phenols is higher than that of alcohols.
- The ionisation of an alcohol and a phenol occurs as follows:
- In alkoxide ion, the negative charge is localised on oxygen, while in phenoxide ion, the charge is delocalised.
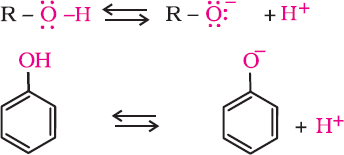
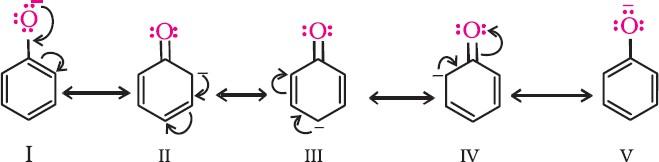
- The delocalisation of the negative charge makes the phenoxide ion more stable and favours the ionisation of phenol. Although there is charge delocalisation in phenol, its resonance structures have charge separation due to which the phenol molecule is less stable than the phenoxide ion.
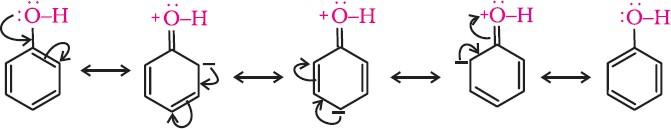
- In substituted phenols, the presence of electron-withdrawing groups such as the nitro group enhances the acidic strength of phenol. On the other hand, electron-releasing groups, such as alkyl groups, decrease the acidic strength. It is because electron-withdrawing groups lead to effective delocalisation of the negative charge in the phenoxide ion.
- Esterification
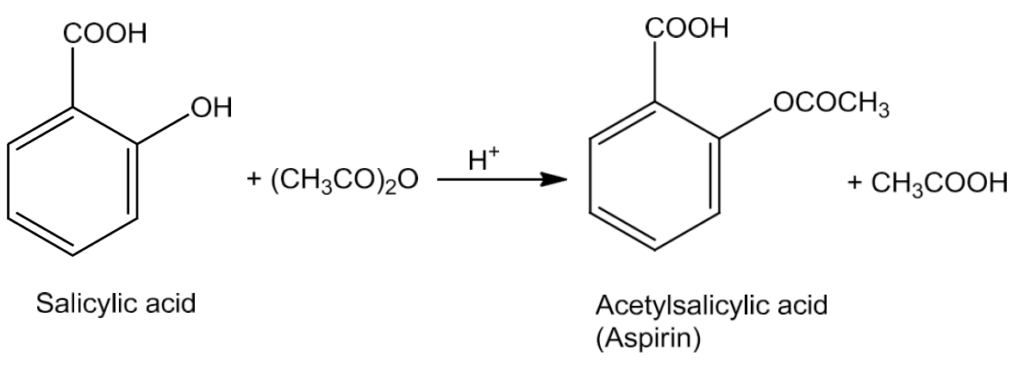
- Esters are formed when alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides.
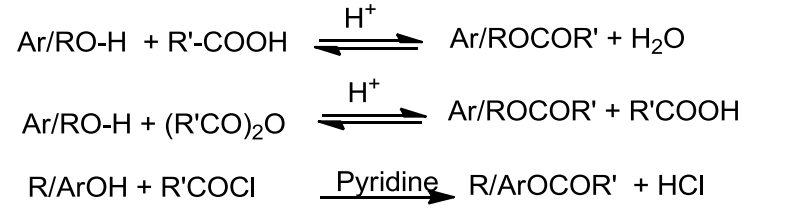
- In case of acid chloride, the reaction is carried out in the presence of base called pyridine to neutralise the HCl formed and to shift the equilibrium to the right.
- Reactions involving cleavage of Carbon-Oxygen(C-O) bond in alcohols
Only alcohols show reactions involving cleavage of C-O bond. Phenols exhibit this type of reaction only with zinc.
- Reaction with hydrogen halides
Alcohols on treatment with hydrogen halides form alkyl halides.
ROH + HX → R-X + H2O
How to distinguish between Primary, Secondary and Tertiary Alcohols?
Lucas reagent test
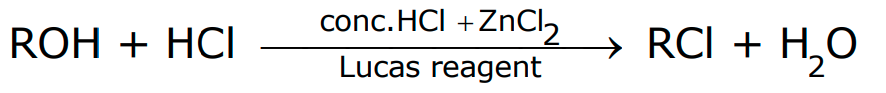
- If it is a primary alcohol, then no turbidity appears at room temperature. Turbidity appears only on heating.
- If it is a secondary alcohol, then turbidity appears in 5 minutes.
- If it is a tertiary alcohol, then turbidity appears immediately.
- Reaction with Phosphorus trihalides
Alcohols get converted into alkyl bromides on treatment with PBr3.
3R-OH + PBr3 → 3R-Br + H3PO3
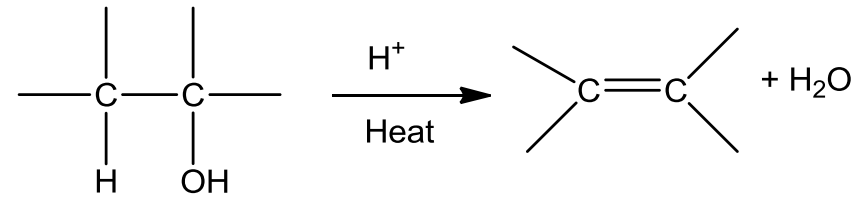
- Dehydration
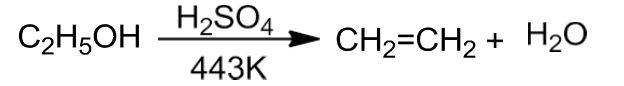
- Alcohols undergo dehydration to form alkenes in the presence of conc. H2SO4 or H3PO3 or catalysts such as anhydrous zinc chloride or alumina.
- Primary alcohol undergoes dehydration by heating it with conc. H2SO4 at 443K.
- Secondary and tertiary alcohols undergo dehydration in milder conditions.
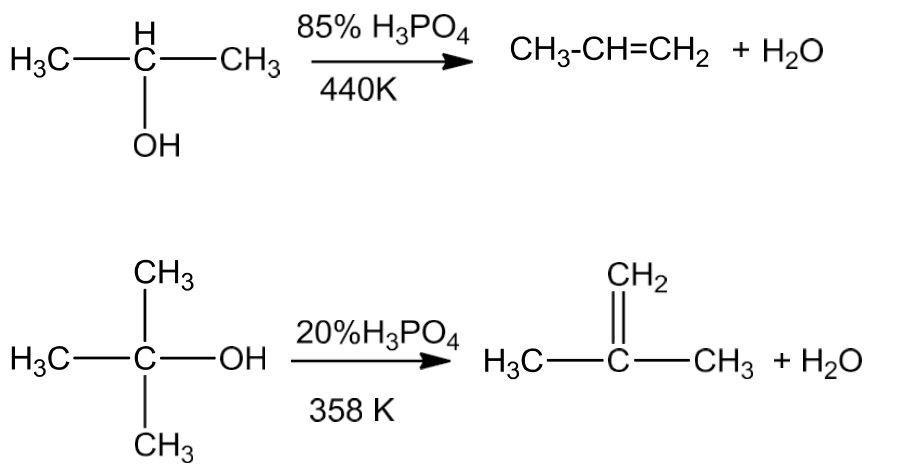
- Thus the ease of dehydration of alcohols follows the order:
Tertiary > Secondary > Primary
- Oxidation
- The oxidation of alcohols results in the formation of a carbon-oxygen double bond with the cleavage of an O-H and C-H bonds. The reaction is known as dehydrogenation reaction as it involves loss of dihydrogen from an alcohol
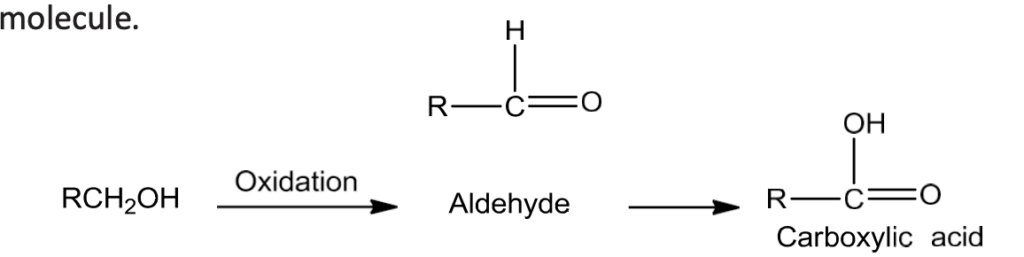
- Use of strong oxidising agents like acidified KMnO4 is done to obtain carboxylic acids from alcohols directly. CrO3 in anhydrous medium is used for obtaining aldehydes.
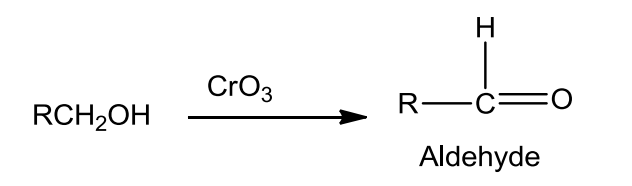
- Pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl is a better oxidizing agent for oxidation of primary alcohols to aldehydes in good yield.

- CrO3 is used to oxidize secondary alcohols to ketones.
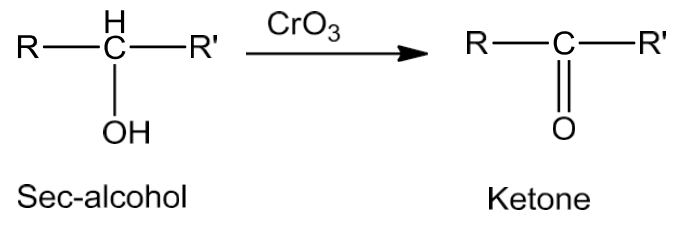
- Tertiary alcohols do not undergo oxidation reaction. In presence of strong oxidizing agents (KMnO4) and elevated temperatures, cleavage of C-C bonds takes place and a mixture of carboxylic acids containing lesser number of carbon atoms is formed.
- On passing vapours of a primary or a secondary alcohol over heated copper at 573K, dehydrogenation takes place and an aldehydes or a ketone is formed whereas tertiary alcohols undergo dehydration.
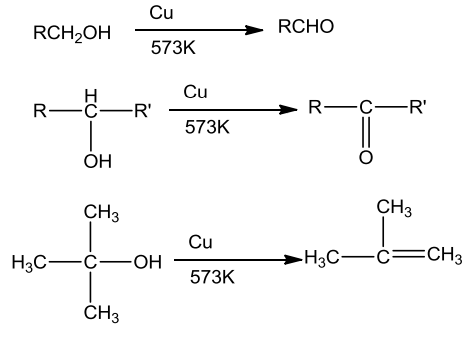
Characteristics of Phenols
- Phenols show electrophilic substitution reactions.
- The –OH group activates the benzene ring towards electrophilic substitution and also directs the incoming group to ortho and para positions in the ring as these positions become electron rich due to the resonance effect caused by –OH group.
- Nitration
Phenol on treatment with dil.HNO3 at low temperature yields a mixture of ortho and para nitrophenols.
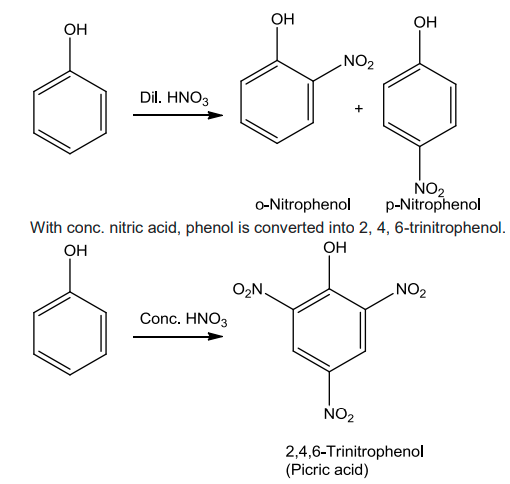
- Halogenation
- Bromine in CHCl3 or CS2
Monobromophenols are formed when phenol is treated with bromine in CHCl3 or CS2 at low temperature.
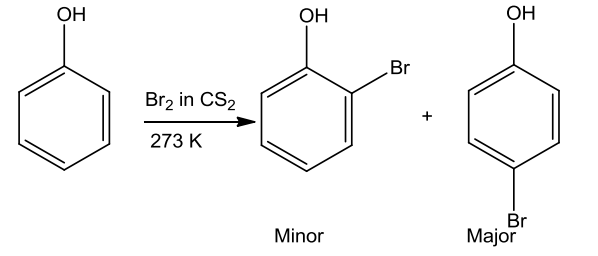
- Action of Bromine water
On treating phenol with bromine water, a white precipitate of 2, 4, 6-tribromophenol is formed.
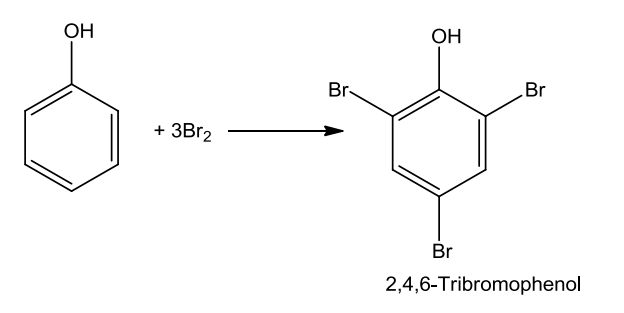
- Kolbe’s Reaction
Phenols on treatment with NaOH produces phenoxide ion which is even more reactive than phenol towards electrophilic aromatic substitution and therefore it undergoes electrophilic substitution with carbon dioxide. Ortho hydroxybenzoic acid is obtained as the main product.
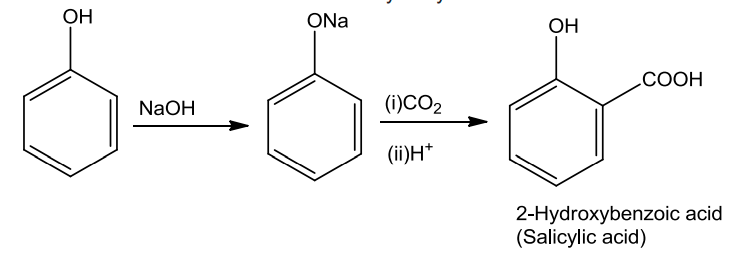
- Reimer-Tiemann Reaction
Phenols on treatment with chloroform in the presence of NaOH, a –CHO group is introduced at ortho position of benzene ring. The substituted benzal chloride formed as intermediate on hydrolysis with alkali produce salicylaldehyde.
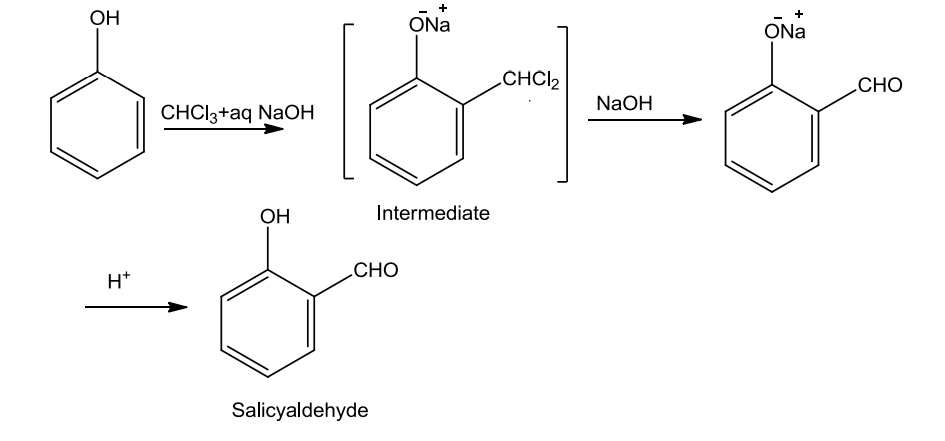
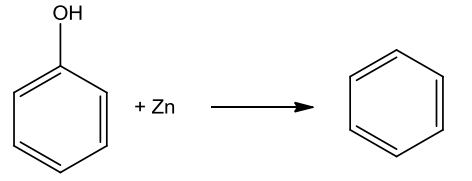
- Action of Zinc dust
Phenol on heating with zinc dust produces benzene.
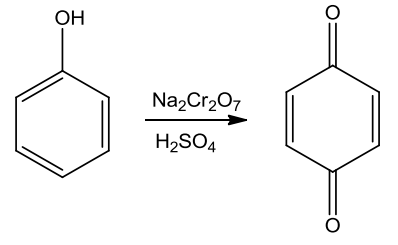
- Oxidation
Phenols on oxidation with chromic acid gives out conjugated diketone known as benzoquinone.
Some Commercially Important Alcohols
Methanol and ethanol are two commercially important alcohols.
Methanol
- Methanol also known as ‘wood spirit’ was produced by destructive distillation of wood.
- Now methanol is produced by catalytic hydrogenation of carbon monoxide at high pressure and temperature in the presence of ZnO-Cr2O3 catalyst.
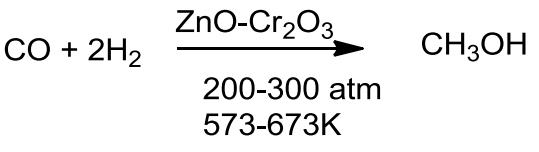
Ethanol
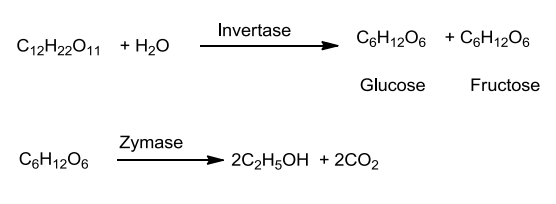
- It is commercially obtained by fermentation from sugars.
- The sugar in molasses, sugarcane or fruits like grapes is converted to glucose and fructose in the presence of an enzyme invertase.
- Glucose and fructose undergo fermentation in the presence of another enzyme, zymase, which is found in the yeast.
Preparation of Ethers
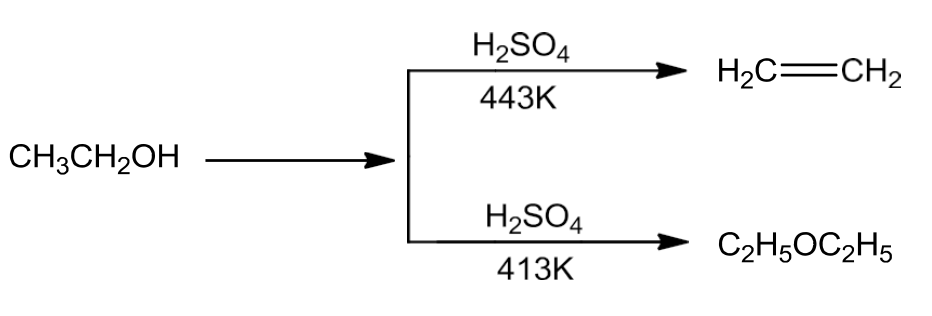
- By Dehydration of Alcohols
- Alcohols on dehydration with protic acids like H2SO4, H3PO4 give alkene or ether depending on the reaction conditions.
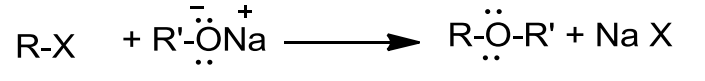
- Williamson Synthesis
- This method is used for the preparation of symmetrical and unsymmetrical ethers.
- In this reaction, an alkyl halide is allowed to react with sodium alkoxide.
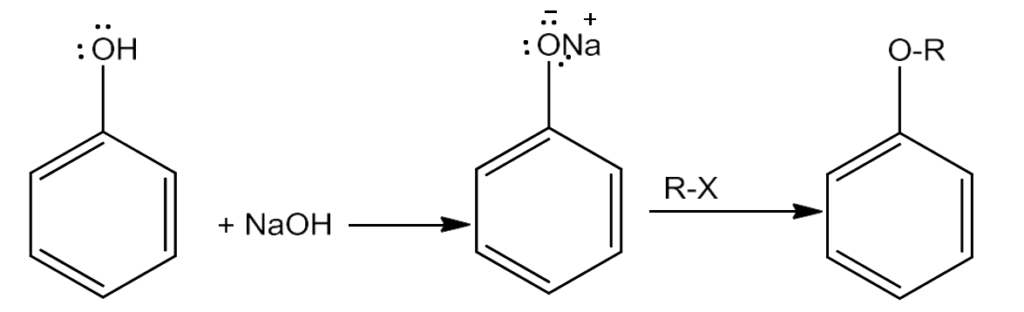
- Phenols can also be converted into ethers using this method. In this, phenol is used as the phenoxide moiety.
Physical Properties of Ethers
- Miscibility
Miscibility of ethers with water resembles those of alcohols of the same molecular mass. This is because similar to alcohols; oxygen of ether can also form hydrogen bonds with the water molecule.
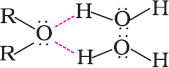
- Boiling points
Ethers have much lower boiling points than alcohols. This is due to the presence of hydrogen bonding in alcohols. Hydrogen bonding is absent in ethers.
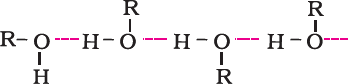
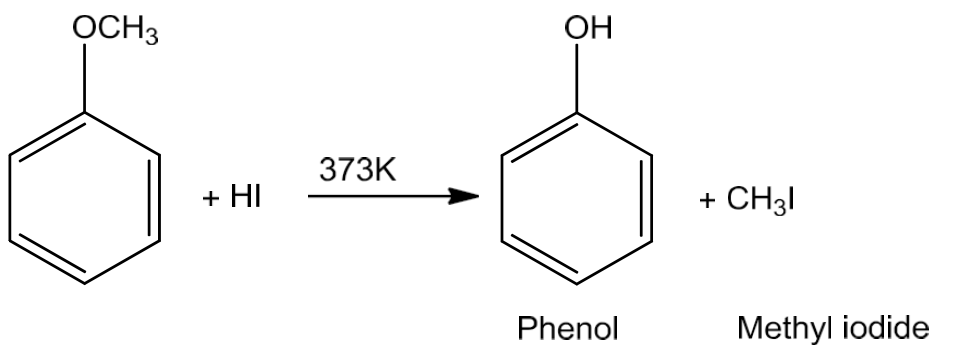
Chemical Properties of Ethers
- Cleavage of C–O bond in ethers
- Since ethers are least reactive of the functional groups, the cleavage of C-O bond in ethers takes place in excess of hydrogen halides.

- The cleavage of ethers with two different alkyl groups also takes place in the same manner.

- In phenolic ethers, the cleavage occurs with the formation of phenol and alkyl halide.
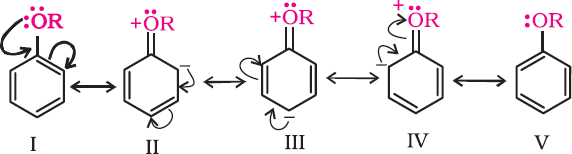
- Electrophilic Substitution
The alkoxy group (-OR) is ortho, para directing and activates the benzene ring for aromatic substitution.
- Halogenation
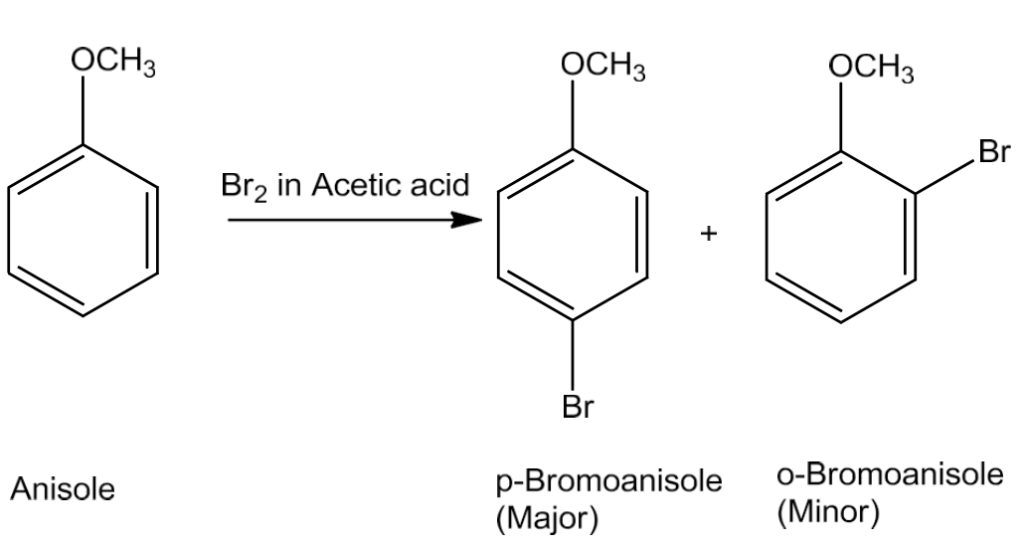
Phenyl alkyl ethers undergo halogenation reaction.
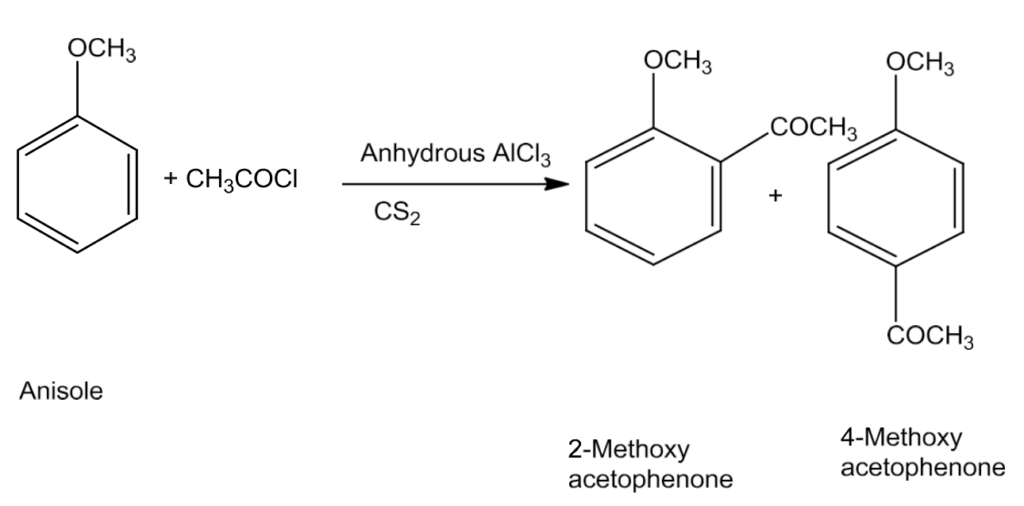
- Friedel-Crafts reaction
In this reaction the alkyl groups and acyl groups are introduced at ortho and para position by treating anisole with alkyl halide and acyl halide in the presence of anhydrous chloride as catalyst.
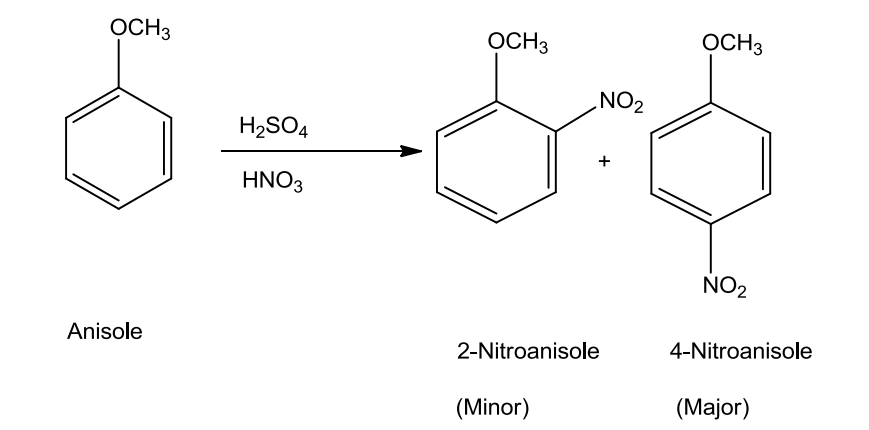
- Nitration
Anisole on treating with a mixture of sulphuric acid and nitric gives a mixture of ortho and para nitroanisole.
Classification of alcohols
We can classify alcohols on the basis of different factors:
Number of hydroxyl groups attached , hybridization ,number of alkyl groups attached to alpha carbon (alpha carbon is that which has functional groups attached to it )
On the basis of number of -OH (hydroxyl ) group attached we have :
Monohydric alcohols: They are those that have only one hydroxyl group attached.
Example: methanol (CH3OH) ethanol (C2H5OH) and more
Dihydric alcohols: They have two hydroxyl groups attached.
Example: ethylene-glycol
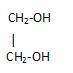
Polyhydric alcohol: That have three or more hydroxyl groups in it.
Example glycerol
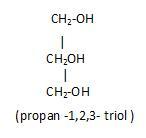
(2). Based on the type of hybridization:
It can be sp3 like ethanol – CH3 – CH2 – OH
Another example :
It can be sp2 like Benzyl alcohol-
In this second carbon is alpha carbon and that is sp3
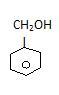
(Benzyl alcohol)
Allyl alcohol CH2 = CH – CH2 OH (In it also alpha carbon is sp3 hybridised ).
Another type of hybridization they possess is sp2
Example: Phenol C6H5OH, Vinyl alcohol CH3 – CH2 = CH – OH etc
(c) Classification is on the basis of primary secondary or tertiary carbon atom (alpha carbon)
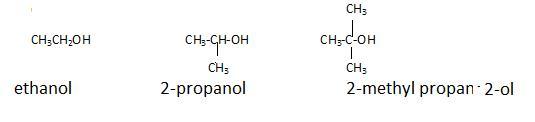
SSS
10 2 30
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
