MCQ Questions for Class 12 Physics Chapter 12 Download Free PDF: CBSE RBSE and NCERT Physics class 12 MCQs for Chapter 12 “Atoms” is very important for the students who want to get good marks. Neet MCQs

MCQ Questions for Class 12 Physics Chapter 12 Atoms with PDF
Question 1.
A spectral line is emitted when an electron
(a) jumps from lover orbit to higher orbit.
(b) jumps from higher orbit to lower orbit.
(c) rotates in a circular orbit.
(d) rotates in an elliptical orbit.
Answer: (b) jumps from higher orbit to lower orbit.
Question 2.
The ionisation potential of hydrogen is 13.6 V. The energy of the atom in n = 2 state will be
(a) -10.2 eV
(b) -6.4eV
(c) – 3.4 eV
(d) – 4.4 eV
Answer: (c) – 3.4 eV
Question 3.
At the time of total solar eclipse, the spectrum of solar radiation would be
(a) a large number of dark Fraunhoffer lines
(b) a small number of dark Fraunhofer lines.
(c) All Fraunhofer lines changed into brilliant colours.
(d) None of these.
Answer: (c) All Fraunhofer lines changed into brilliant colours.
Question 4.
The adjoining figure indicates the energy levels of a certain atom when the system moves from 2 E to E level, a photon of wavelength λ is emitted. The wavelength of photon produced during its transition from 4E3 to E is
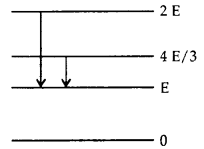
Answer: (d)
Question 5.
A hydrogen atom is in the p-state. For this, values of J are
(a) 52, 32, 12
(b) 32, 12
(c) –12,12, 32
(d) –12, −32
Answer: (b) 32, 12
Question 6.
Energy levels A, B, C of a certain atom correspond to increasing value of energy i.e., EA > EB > EC. If λ1, λ2 and λ3 are the wavelengths of radiation corresponding to transition C to B, B to A and C to A respectively, which of these of the following is correct?
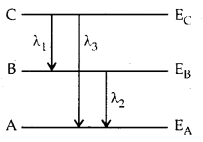
Answer: (b)
Question 7.
In Rutherford’s scattering experiment with gold foil, 232 counts per minute are observed at an angle of 60°. The number of counts/min. at an angle of 120° will be
(A) 232
(b) 116
(c) 26
(d) 52
Answer: (c) 26
Question 8.
In an atom, the two electrons move round the nucleus in circular orbits of radii R and 4R. The ratio of the times taken by them to complete one revolution is
(a) 1/8
(b) 1/4
(c) 4
(d) 8
Answer: (a) 1/8
Question 9.
The ratio of the energies of the hydrogen atom in its first to second excited state is :
(a) 1/4
(b) 4/9
(c) 9/4
(d) 4.
Answer: (c) 9/4
Question 10.
In Bohr’s model of the hydrogen atom, the ratio between the period of revolution of an electron in the orbit n = 1 to the period of revolution of electron in the orbit n = 2 is
(a) 1/2
(b) 1/4
(c) 1.8
(d) 2.
Answer: (c) 1/8
Question 11.
According to Bohr’s theory, the radius of electron in an orbit described by the principal quantum number n and the atomic number Z is propotional to :
Answer: (d)
Question 12.
The electron in hydrogen atom jumps from the 3rd orbit to second orbit. The wavelength X of the emitted radiations is
Answer: (a)
Question 13.
To explain fine structure of spectrum of hydrogen atom, we must consider.
(a) a finite size of nucleus.
(b) the presence of neutrons in the nucleus.
(c) spin angular momentum.
(d) orbital angular momentum.
Answer: (b) the presence of neutrons in the nucleus.
Question 14.
The ratio of the energy of the electron in first orbit to that in the second orbit is
(a) ¼
(b) ½ R
(c) 2
(d) 4
Answer: (d) 4
Question 15.
When an electron jumps from some outer orb it to the innermost orbit in the hydrogen atom, the spectral line belongs to
(a) Lyman series
(b) Balmer series
(c) Paschen series
(d) Pfund series
Answer: (a) Lyman series
Question 16.
How does the energy difference between two consecutive energy levels vary on the quantum number n increases?
(a) does not change
(b) decrases
(c) increases
(d) may increase or decrease.
Answer: (b) decrases
Question 17.
According to classical theory, Rutherford atom is
(a) stable
(b) unstable
(c) metastable
(d) semistable
Answer: (b) unstable
Question 18.
For an electron orbit to be non-radiating, it should be
(a) such that the angular momentum should be integral multiple of h.
(b) circular in nature
(c) elliptical in nature
(d) none of these
Answer: (a) such that the angular momentum should be integral multiple of h.
Question 19.
Which of the following type ot radiation is not emitted by the electronic structure of atoms :
(a) X-rays
(b) Visible light
(c) γ-rays
(d) Ultraviolet light.
Answer: (c) γ-rays
Question 20.
If the electron in hydrogen atoms is excited to n = 5 state, the number of different frequencies of radiation which may be emitted is:
(a) 4
(b) 10
(c) 8
(d) 5
Answer: (b) 10
Question 21.
The ratio of the angular momentum of an electron in first orbit to that in the second orbit is
(a) 12
(b) 14
(c) 4
(d) 2
Answer: (a) 12
Question 1
………………… of the electron in the orbit signifies that the electron and nucleus is a bound system.
Answer: Negative energy.
Question 2.
The ………………… lies in the infrared region of the spectrum.
Answer: Paschen series.
Question 3.
Lyman series lies in the ………………… region of spectrum and Balmer series lies in the ………………… of the spectrum.
Answer: Ultraviolet, visible region.
Question 4.
The difference of energy levels goes on ………………… as we move towards higher energy levels.
Answer: decreasing.
Question 5.
Separation between the orbits goes on ………………… as we move towards higher orbits.
Answer: increasing.
Question 6.
The radius of the first orbit of hydrogen atom is ………………… times the radius of first orbit of a H-like helium atom.
Answer: two.
Question 7.
The minimum energy required to excite a hydrogen atom from its ground state is …………………
Answer: 10.2 eV.
Question 8.
In a hydrogen atom, the electron moves in an orbit of radius 0.5 Å making 1016 revolutions per second. The magnetic dipole moment associated with the orbital motion of the electron is …………………
Answer: 256 × 10-23 Am².
Question 9.
Band spectrum is produced by the substance in ………………… state.
Answer: molecular.
Question 10,
Rutherford’s a-particle scattering experiment shows the existence of a ………………… charged nucleus of ………………… size located at the …………………
Answer: Positively, very small, centre of the atom.
Question 11.
The maximum number of photons emitted when an electron jumps from an energy level n = 4 to n = 1 is …………………
Answer: 6.
Question 12.
The radius of Bohr’s first orbit is a0. The electron in nth orbit has a radius …………………
Answer: n² a0
Question 13.
The kinetic energy associated with an electron decreases with an ………………… in the radii of the orbits.
Answer: increase.
Question 14.
For a given projectile and target, the distance of closest approach ………………… with increase in K.E. of the projectile.
Answer: decreases.
Question 15.
In scattering of α-particles by nucleus, the distance of closest approach depends upon the charges of ………………… and ………………… as well as ………………… of α-particle.
Answer: Projectile, target nucleus, kinetic-energy.
Question 16.
The ionisation energy of hydrogen atom is E. When the electron in a hydrogen atom jumps from the state n = 1 to the state n = 2, the energy absorbed by it is …………………
Answer: 3E / 4
Question 17.
The energy of the atom goes on ………………… as we go to higher excited states.
Answer: increasing.
Question 18.
From Bohr’s theory, when an electron jumps from higher energy orbit to second orbit, the spectral lines that occur belong to ………………… series.
Answer: Balmer.
Question 19.
When a hydrogen atom is raised from the ground state to an excited state, them P.E ………………… and Kinetic energy …………………
Answer: increases, decreases.
Question 20.
If elements with principal quantum number n > 4 were not existed in nature then the number of possible electrons would be …………………
Answer: 60.
MCQ Questions for Class 12 Physics Chapter wise
mcqs of physics class 12 chapter 12, mcq of physics class 12 pdf, physics class 12 mcq chapter wise, online mcq test physics class 12 mod education, 12th physics mcq pdf download, physics mcq with answers pdf, mcq questions for class 12 physics chapter 12, mcqs of physics class 12 chapter 12 pdf
