NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 5, “Surface Chemistry,” is a study material package designed to help students understand the principles of surface chemistry, the study of chemical reactions that occur at the interface of two phases, such as solids and liquids, or liquids and gases.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including adsorption, absorption, colloids, emulsions, and micelles. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the types of adsorption, such as physical and chemical adsorption, to the factors affecting adsorption and the properties of colloids. It also includes questions on the different types of emulsions and their uses, as well as the structure and properties of micelles.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the different types of adsorption, the properties of colloids, and the types of emulsions and their uses.
NCERT Solution / Notes Class 12 Chemistry Chapter 5 Surface Chemistry with mind map PDF Download
Introduction
Surface Chemistry deals with the study of physical and chemical phenomena occurring at the boundary (interface) separating two bulk phases.
The bulk phase can be a pure compound or a solution.
The bulk phases may be solid–liquid, solid–gas, solid–vacuum, liquid – gas etc.
Let us consider a simple example of a dirty shirt. The dirt stays in the surface of the fabric and the study of phenomena occurring at the interface between the fabric and the dirt is surface chemistry, in simple words.

Adsorption and Absorption
The dirt staying on the surface of the skin as a layer is termed as adsorbtion.
Now consider applying soap solution to the shirt containing dirt. The soap solution is absorbed by the fabric and does not stay on the fabric as a layer. This is called absorption.
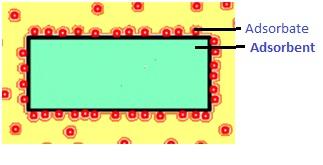
Adsorption: Adsorption is the phenomenon of attracting and retaining molecules of a substance on the surface of a solid (or liquid) resulting as a higher concentration of molecules only on the surface.
- Adsorbent- the surface on which adsorption takes place
- Adsorbate- the substance which is adsorbed

Example- Water vapour adsorbed by silica gel
- Adsorbent- Silica gel
- Adsorbate- Water vapour
Differences between Adsorption and Absorption
| ADSORPTION | ABSORPTION |
| Surface phenomenon- concentration of the adsorbate increases only on the surface | Bulk phenomenon- concentration is uniform throughout the solid |
| Exothermic process- heat is released | Endothermic process- heat is absorbed |
| It is favoured by low temperature | It is not affected by temperature |
| Eg- Chalk stick dipped in ink adsorbs the colour of the ink but when you break the piece of chalk, its core still remains white | Eg- When anhydrous CaCl2 absorbs water vapour, it becomes wet and pasty |
Mechanism of Adsorption
Adsorption occurs because the particle on the surface and the particle in the bulk of the adsorbent are not in the same environment. That is, the net force acting on them is not the same.
The particle on the surface has unbalanced forces acting on it which are also called residual attractive forces
Due to these forces, the surface particles of the adsorbent attract the adsorbate particles
During adsorption, there is always a decrease in the residual attractive forces of the surface. That is, the energy of the surface decreases and this appears as heat. This is called the heat of adsorption
The amount of heat evolved when one mole of adsorbate is adsorbed on the adsorbent surface is called enthalpy of adsorption
Adsorption is always exothermic and the enthalpy change, ΔH is always negative
When the adsorbate molecules are adsorbed on the surface of the adsorbent, their freedom of movement becomes restricted and hence ΔS the entropy decreases
We know that Gibbs free energy, ΔG= ΔH –TΔS.
For adsorption to be spontaneous, ΔG must be negative. This can happen if ΔH has a significantly high negative value as –TΔS is positive.
As the adsorption continues, ΔH becomes less and less negative till it becomes equal to TΔS and ΔG becomes zero. At this point, equilibrium is attained.
Factors affecting adsorption of Gases by Solids
- Nature and Surface area of adsorbent:
- The same gas is adsorbed by different solids at different extents even at the same temperature.
- Greater the surface area, greater is the volume of gases adsorbed.
- Nature of the gas being adsorbed:
- Different gases are adsorbed are adsorbed to different extents even by the same solid.
- As the critical temperature of a gas increases, it is easier to liquefy and it is also more readily adsorbed.
- Reason- Higher the critical temperature, the easier it is to liquefy the gas as greater are the intermolecular forces to attraction between the molecules of the gas. For such a gas, the intermolecular forces of attraction are greater on the surface of the adsorbent and thus, the adsorption will be more.
- Temperature: As temperature increases, adsorption decreases
- Pressure: At constant temperature, the adsorption of a gas increases with increase in pressure.
- Activation of the solid adsorbent: It means increasing the adsorbing power of the adsorbent.
It can be done by-
Making the surface of the adsorbent rough- It can be done by rubbing the surface or chemical action or by depositing fine metal particles on the surface by electroplating
By dividing the adsorbent into small pieces or grains- It increases the surface area but this method has a practical limitation. If the particles are too fine like powder, the adsorption of the gas will become difficult.
By removing the already adsorbed gases
Types of Adsorption
| Physisorption | Chemisorption |
| Occurs due van der Waals forces | Caused by chemical bond formation |
| Not specific in nature that is all gases are adsorbed on the surface to an extent | Highly specific in nature |
| Reversible | Irreversible |
| More easily liquefiable gases are adsorbed more readily | Gases which can react with the adsorbent show chemisorption |
| Enthalpy of adsorption is low(20-40 kJ) | Enthalpy of adsorption is high (80-240 kJ) |
| It decreases with increase in temperature. Favours low temperature | It increases with increase in temperature. Favours high temperature |
| It does not need any activation energy | It does require activation energy. |
| It results into multimolecular layers on adsorbent surface under high pressure | It results into a unimolecular layer. |
Adsorption isotherms
The variation in the amount of gas adsorbed by the adsorbent at constant temperature with change in pressure is shown by a curve called adsorption isotherm.
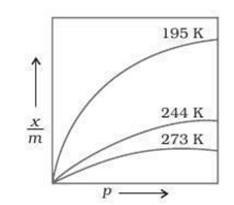
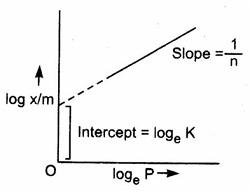
Freundlich adsorption Isotherm:
Freundlich proposed this relation to show a relation between the extent of adsorption and pressure.

If there are solutions involved, the above equations become
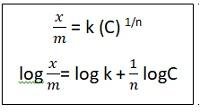
Where x -> amount of adsorbate
m-> mass of adsorbent
p-> pressure
C-> concentration of adsorbate
K and n->constants , n>1 always
Looking at equation 2, as we plot log x/m versus log p on a graph, we get a straight line with slope=1/n and y- intercept= log k.
Applications of adsorption
Production of high vacuum- Remaining traces of air in a vessel already evacuated by vacuum pump can be adsorbed by charcoal to create high vacuum
Control humidity – Silica and aluminium gels can adsorb moisture and remove humidity
Gas masks- Gas masks consist of activated charcoal or mixture of adsorbents and are used to breathe in coal mines
Removing coloured substances from solutions- This is used in chromatographic analysis.
Separation of inert gases- Different inert gases are adsorbed to different extents on coconut charcoal
Heterogeneous catalysis- When gaseous reactants are adsorbed on the surface of a solid catalyst, the concentration of the reactants on the surface increases and thus, the rate of reaction also increases ( adsorption theory). Eg- Using finely divided Nickel in the hydrogenation of vegetable oils
Adsorption indicators- Many dyes have become useful due to adsorption. These dyes have been introduced as indicators especially in precipitation titrations. Eg- KBr is easily titrated with AgNO3 using eosin as the indicator
Froth floatation process- When sulphide ore is shaken with pine oil and water, the ore particles are adsorbed on the froth that floats and the gangue prticles ( like silica, mud) settle down in the tank. This process is used in the concentration of sulphide ores
Chromatographic analysis- The selective adsorption of some substances by a solution helps us separate components of a mixture. Example- All the dyes in ink
Curing Diseases- Some drugs can adsorb the germs on them and hence, kill them saving us from diseases
Properties of Colloids
Physical Properties
- Heterogeneous character:
- Colloidal sols form heterogeneous mixtures which contain particles of the dispersed phase and the dispersion medium.
- The heterogeneous character of this sol can be explained more conveniently by phenomena such as the Tyndall effect, electrophoresis and electro-osmosis.
- Stability:
- Colloidal sols are stable. The sol which contains larger particle size settles very slowly.
- Filterability:
- The size of the pores of ordinary filter paper is large and ordinary filter paper cannot be used for the separation of the dispersed phase, because the particle size of colloidal sols is very small and particles can easily pass through.
- Instead of ordinary filter paper, animal membrane or parchment filter paper can be used because the pore size is very small; hence, the colloidal particles cannot pass through.
- Visibility and Colour:
- Colloidal particles are very small; hence, they cannot be seen by the naked eye or under an ordinary microscope.
- They scatter the light falling on them. The colour of colloidal solutions depends on the wavelength of the light scattered by colloidal particles and on the size and nature of colloidal particles.
- For example, in a gold sol, if the particle size is very small, then it shows red colour, but if its size increases, then the colour changes to purple, then blue and finally gold.
- The colour factor also depends on how light is observed.
Colligative Properties – Osmotic Pressure
- Colloidal particles possess very high molecular mass; hence, the number of moles present in the solution is very small. Therefore, the value of the colligative property will be less as compared to the value obtained by true solutions.
- Some colloids have measurable osmotic pressure which has been determined with a reasonable degree of accuracy. Hence, the osmotic pressure is used to calculate the average molecular mass of colloidal particles.
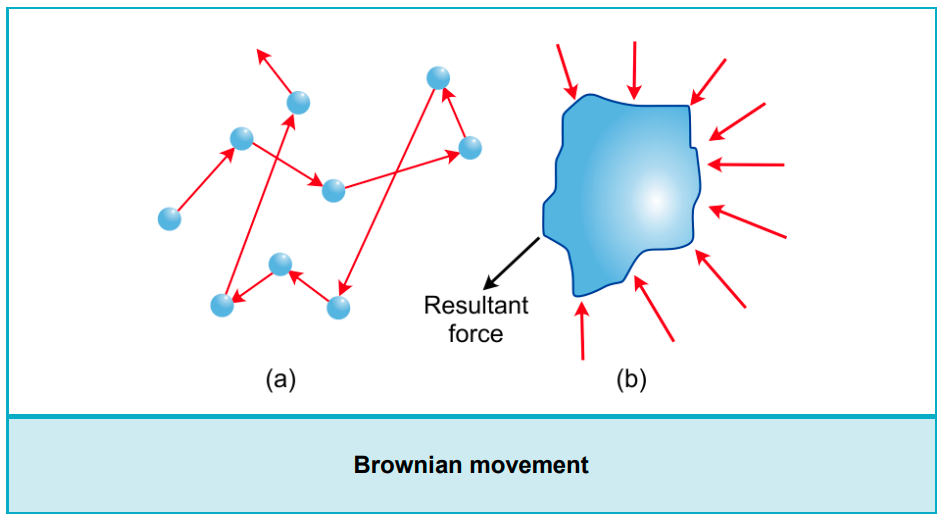
Mechanical Properties – Brownian Movement
- In 1827, Robert Brown observed the movement of pollen grains suspended in water. Like pollen grains, colloidal particles also continuously move in a zigzag manner when seen under an ultramicroscope. Hence, this movement is called Brownian movement.
- Brownian movement does not depend on the nature of the colloid. But it depends on the size of colloidal particles and the viscosity of the sol.
- Smaller the size and less viscosity of sol will give faster movement of particles.
Brownian movement: Continuous zigzag movement of colloidal particles in a colloidal sol.
Cause of Brownian movement:
- The main cause of Brownian movement is the collision of molecules of the dispersion medium with the colloidal particles due to their kinetic motion from all sides with different force.
- Brownian movement depends on the size of colloidal particles. If particles are heavier, then the movement will be slower.
Importance of Brownian movement:
- Brownian movement opposes the force of gravity and does not allow the colloidal particles to settle. Thus, it is responsible for the stability of colloidal particles.
- It is useful in the determination of Avogadro’s number.
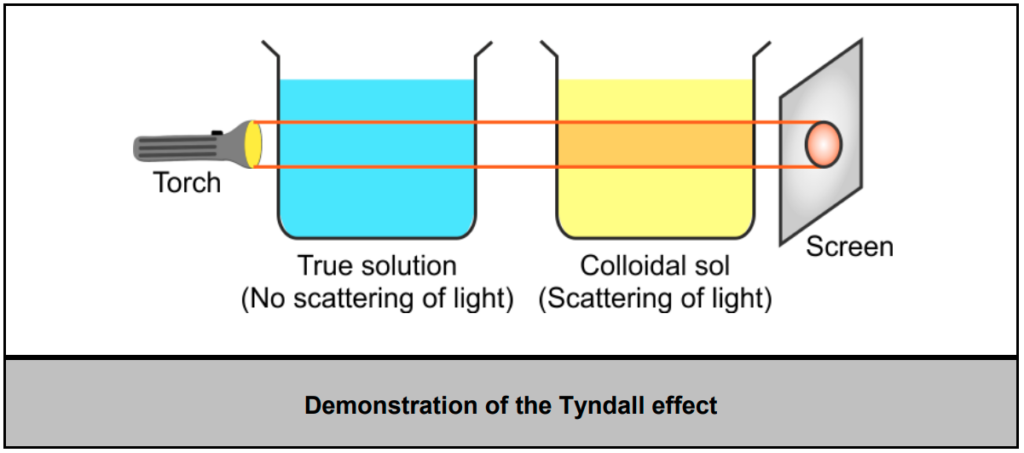
Optical Properties – Tyndall Effect
- In 1969, the scientist Tyndall observed an optical property of colloidal particles called the Tyndall effect. This phenomenon is based on the scattering of light.
- He observed that when a converging beam of light is passed through the colloidal solution placed in a dark room, the path of the beam gets illuminated with bluish light when viewed at right angles to the direction of the passage of light.
- The path of light is visible because of the scattering of light by colloidal particles. This path is known as Tyndall cone.

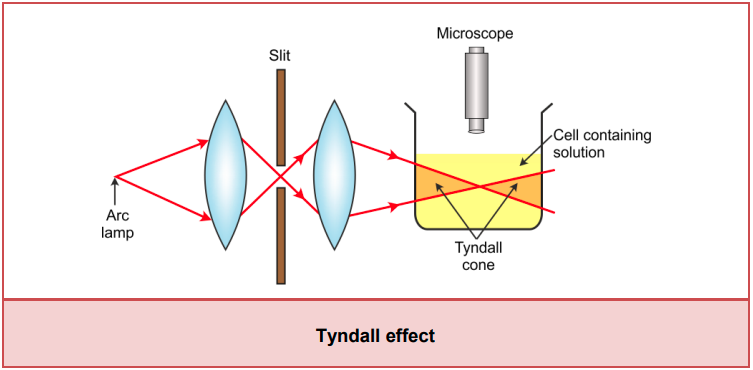
- We can simply explain the Tyndall effect in the laboratory by performing an experiment.
- Take two beakers arranged in linear manner. One containing a colloidal sol and the other containing a true solution. The path of light is incident on both beakers.
- The path of light is visible in a beaker containing a colloidal sol, and no path is observed in another beaker containing a true solution.
- Hence, we can conclude the following conditions for the Tyndall effect to be observed:
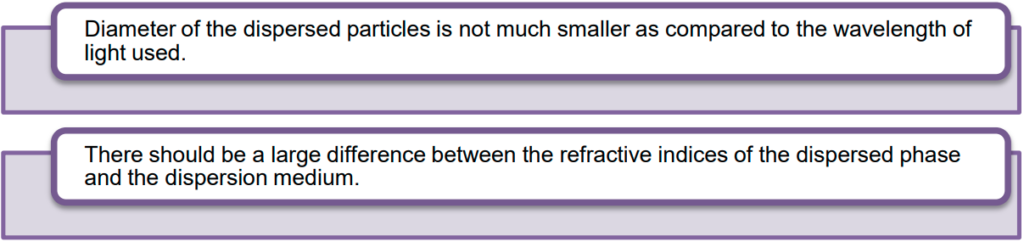
Importance of the Tyndall effect:
- The ultramicroscope was devised in 1903. The working of the ultramicroscope is based on the Tyndall effect.
- A beam of light is focused on the colloidal solution taken in a glass vessel and is observed under the microscope at right angles to the beam.
- The light scattered by each colloidal particle looks bright against the dark background. Thus, the number of colloidal particles can be counted.
- By knowing the volume of the solution, the number of particles per unit volume can be determined.
- It also helps find the average mass of particles.
- The ultramicroscope does not give information about the size and shape of particles as we cannot see the actual particles by it; only the light scattered by the particle is observed.
Electrical Properties
- Stability of colloidal sols – Electrical charge on colloidal particles:
- Colloidal particles in the sol are electrically charged; hence, they are stable. Therefore, these particles never come close to each other. They repel the neighbouring particles to form large non-colloidal particles.
- All the dispersed particles in the colloidal solution carry the same charge, while the dispersion medium has an equal and opposite charge. For example, ferric hydroxide particles are positively charged, while the dispersion medium water is negatively charged.
Origin of electrical charge on colloidal particles:
- An ionic colloid adsorbs ions common to its own lattice during the preparation of the colloidal sol. For example, if a colloidal sol of AgI is prepared by adding KI solution to AgNO3 solution till KI is in slight excess, iodide ions will be adsorbed on the surface of AgI particles giving them a negative charge.

- When a colloidal sol of AgI is prepared by adding AgNO3 solution to KI solution till AgNO3 is in slight excess; Ag+ ions will be adsorbed giving a positive charge to the colloidal particles.
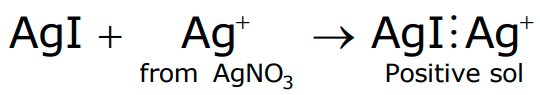
- In both cases, the remaining ions, i.e. K+ and NO −, remain in the dispersion medium and give equal and opposite charge to the dispersion medium.
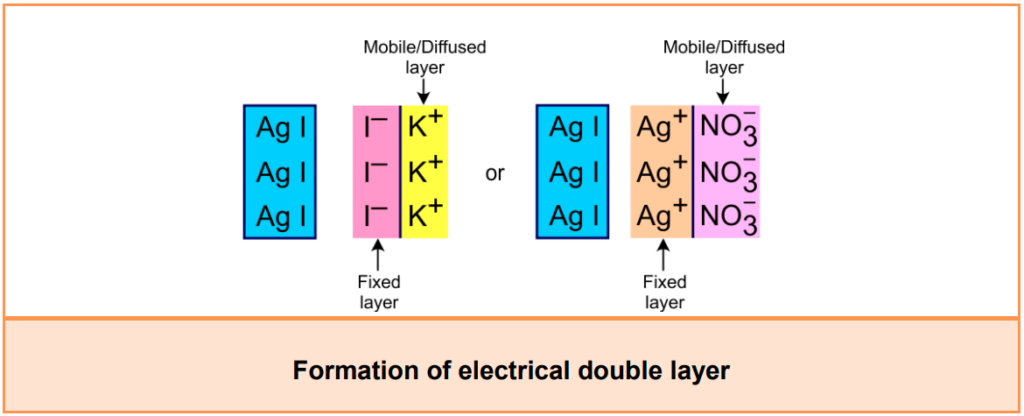
Electrokinetic or Zeta potential:
- When one type of ions of the electrolyte is adsorbed on the surface of the colloidal particles, it forms a fixed layer.
- Then it attacks the counter ions from the medium forming a second layer, which is moving and is known as the diffused layer.
- The double layer of opposite charges thus formed is called the Helmholtz electrical double layer.
- Because of this, a potential difference is created between the fixed layer and the diffused layer, which is known as the electrokinetic potential or Zeta potential.
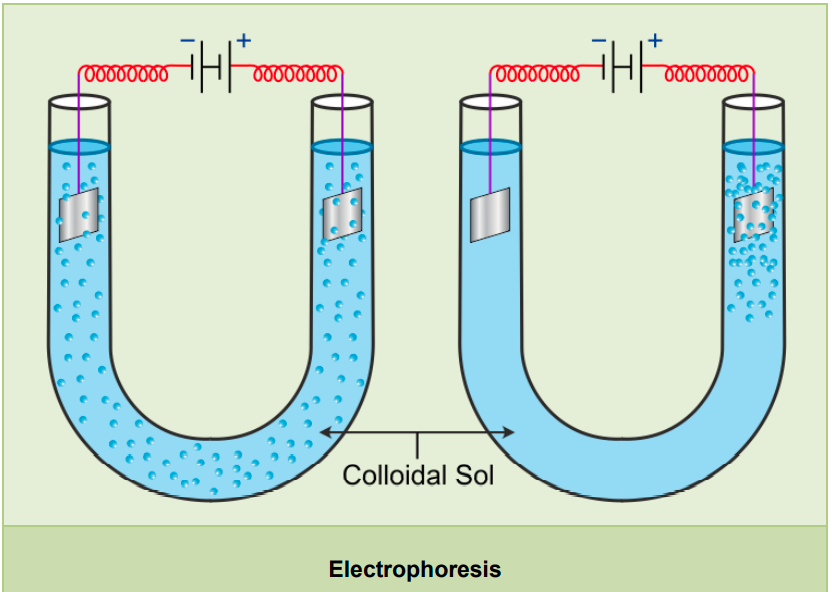
- Electrophoresis or cataphoresis:
- By electrophoresis, we can show the presence of electrical charge.
- It involves the movement of colloidal particles towards one or the other electrode when placed under the influence of an electric field.

- When these colloidal particles reach the oppositely charged electrode, they get neutralized and coagulated.
- Electrophoresis can be used to find the nature of charge on colloidal particles.
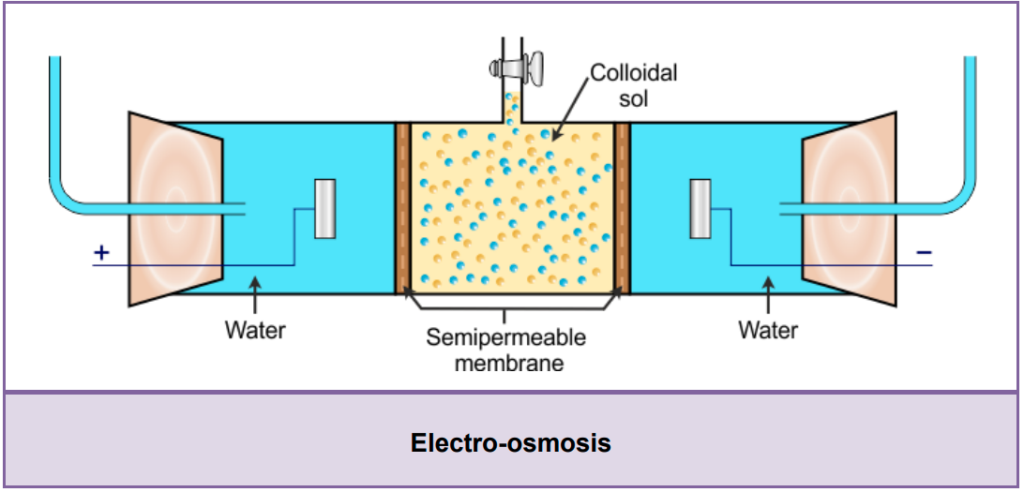
(c) Electro-osmosis:
- When a colloidal solution is placed under the influence of an electric field, the particles of the dispersion medium move towards the oppositely charged electrode. But colloidal particles do not move. This phenomenon is called electro-osmosis.

- Coagulation or flocculation or precipitation:

- When an electrolyte is added to a colloidal solution, the particles of the sol take up the ions which are oppositely charged and get neutralised.
- These ions which are responsible for the neutralisation of charge on the colloidal particles are called coagulating ions or flocculating ions.
- Neutral particles then come together to form larger particles which then settle.
- When the concentration of the electrolyte is very low, the process is called flocculation. The process can be reversed on simple shaking.

Hardy Schulze Law
Main points of this law:

- For example, for the coagulation of negatively charged arsenious sulphide sol, trivalent cations (Al+) are far more effective than divalent cations (Ba2+). This in turn is more effective than monovalent cations (Na+).
- For the coagulation of positively charged ferric hydroxide sol, tetravalent anions [Fe(CN)6]4− are more effective than trivalent anions (PO43-) which are more effective than divalent anions (SO42-)
which are also more effective than monovalent anions (Cl−).
- The coagulating power is inversely proportional to the coagulation or flocculation value, so comparing the relative coagulating power of two electrolytes for the same colloidal solution, we get
Coagulating power of electrolyte 1 Coagulation value of electrolyte 2 Coagulating power of electrolyte 2 Coagulation value of electrolyte 1
Causes of Coagulation
- By electrophoresis:
- Particles of the dispersed phase move towards the oppositely charged electrode and get neutralised.
- When the process is continued for sufficient time, these neutral particles combine and settle.
- By mutual precipitation:
- Oppositely charged sols are mixed in proper proportions to neutralise the charge of each other which causes coagulation of both sols.
- If positively charged ferric hydroxide and negatively charged arsenious sulphide sols are mixed, then both sols get coagulated.
- By prolonged dialysis:
- Stability of a colloidal solution is due to the electrolyte present in it.
- On prolonged dialysis, the electrolyte is completely removed. Thus, the colloidal solution becomes unstable and coagulates.
- By heating or cooling:
- Heating the sol causes coagulation. Example: Coagulation of butter
- When a sol is heated, the adsorbed layer is disturbed because the number of collisions on them by the molecules of the dispersion medium increases, i.e. the charge on the particles decreases; thus, stability decreases and coagulation occurs.
- Sometimes cooling the sol causes coagulation. Example: Coagulation of milk.
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
