NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 1, “The Solid State,” is a comprehensive study material package designed to help students understand the principles and concepts of solid-state chemistry.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including the classification of solids, crystal structures, and the packing of atoms in solids. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the difference between amorphous and crystalline solids to the types of defects in crystals. It also includes questions on the properties of solids, such as electrical conductivity, thermal conductivity, and hardness.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the classification of solids, crystal structures, and the packing of atoms in solids.
NCERT Solution Class 12 Chemistry Chapter 1 The Solid State with Mind Map PDF Download
Introduction: Everything in our surroundings is known as matter that can be categorized into three states.
In our day to day life solids plays a crucial role to pursue different purposes. Different types of solids with different properties are required for different purposes. The constituent particles and the type of bonds between the particles determine the nature of a specific solid. For eg. Bucket or a container used to carry water, utensils used for cooking food, computer, vehicles, electronic gadgets, notebooks, pencils, papers etc. are all solid substances used in our day to day life.

Liquids and gases on the other hand are another state of matter and are also known as fluids due to their ability to flow. They attain the ability to flow due to the free movement of molecules.
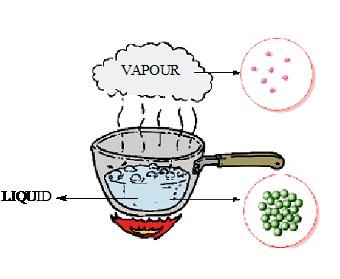
Fig. Particles of liquid (water) are loosely packed than solid and have space between them whereas particles of gas are loosely packed and have excess space between the particles
Characteristics of solid state
- They have definite shape due to strong Intermolecular forces of attraction.
- They have distinct boundaries.
- They have a fixed volume.
- They cannot flow.
- They have negligible compressibility due to negligible distance between the neighbouring molecules.
- They possess a tendency to uphold their shape when exposed to external force.
- They break under force but it is difficult to change their shape so they are rigid.
- They have high density and do not diffuse at all.
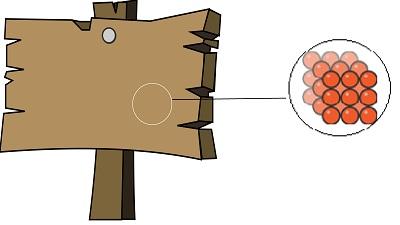
Fig. Particles of solid (Wooden plank) are compact together and have less space between them
Classification of Solids
Solids are classified on the basis of two different parameters i.e.,
- Packing of constituent particles
- Forces of attraction among constituent particles.
Classification on the basis of packing
1. Crystalline Solids
These are the solids in which the constituent particles (atoms, ions or molecules) are arranged in a regular, three dimensional orderly arrangement, which gets repeated throughout. Hence, these are also called as long-range order solids. Quartz, diamond, Boron Nitride, NaCl, ZnS, CsCl etc.
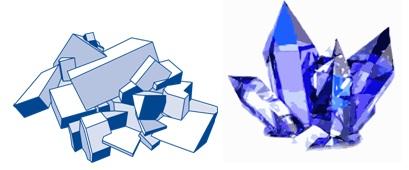
2. Amorphous Solids
Constituent particles are randomly scattered with, no regular arrangement of particles. Therefore they do not have any definite shape or form. Rubber, glass, plastic etc. are commonly known as amorphous solids.
Classification on the basis of Nature of Intermolecular Forces
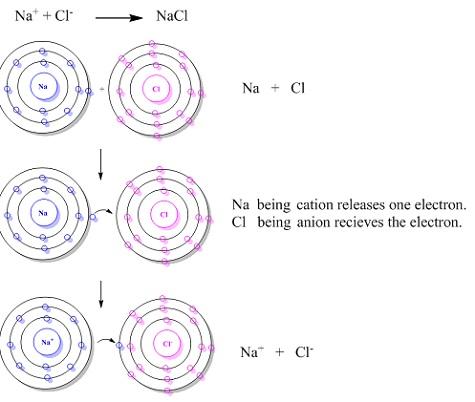
(i) Ionic Solids
There is a regular arrangement of positively and negatively charged ions throughout the solid where ions are held together by strong coulombic or electrostatic forces. These solids are very hard and brittle and have very high melting points. In solid state, as ions are not free to move, hence they are insulators but in molten state or in aqueous state, it’s ions become free to move and it becomes conductor. Ionic solids have high enthalpies of vaporisation. Ex- LiF, NaCl, KNO3, MgO, etc.
(ii) Metallic Solids
Metal cores and a sea of mobile electrons are the constituents of metallic solids. Each metal atom contributes one or more electrons towards sea of electrons. These electrons are evenly spread out throughout the crystals and weak forces of attraction or metallic bond binds together kernels and sea of electrons.
Metallic crystals may be hard as well as soft having moderate enthalpies of fusion. Mobile sea of electrons is responsible for many properties of metals such as malleability (can be beaten into thin sheets), ductility (can be drawn into wires), metallic lustre, thermal conductivity and electrical conductivity etc. Ex- Copper, Iron, Nickel etc.
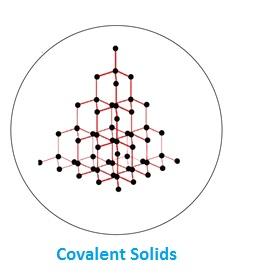
(iii) Covalent Solids (Network Solid)
In these atoms are bonded together by covalent bond formation throughout the crystal. It means there is a continuous network of covalent bonds forming a giant three dimensional structure or giant molecule. Covalent bonds are strong and directional in nature. These solids are very hard, brittle and very high melting point. Due to absence of any free electrons or ions they are insulators. Their enthalpies of fusion are very high. Ex- Diamond, Graphite, Boron Nitride, Silicon Carbide (SiC) etc.
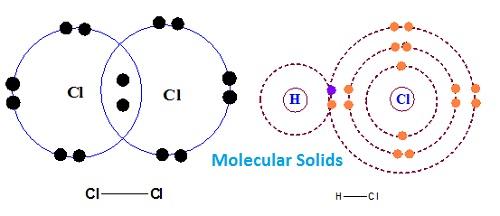
(iv) Molecular Solids
Their molecules are held together by dispersion forces, London forces, dipole-dipole forces or hydrogen bonds. Either atoms or molecules are bonded together by weak dispersion forces or London forces. These are non-conductor soft solids with low m.p. and low enthalpies of vaporisation. They are volatile in nature hence, at room temperature and pressure they are available in liquid or gaseous state. Ex- Iodine, Solid H2 and CO2 (dry ice). Naphthalene, Camphor etc.
Crystal Lattices and Unit Cells
(i) Crystal Lattices
In a crystalline solid, constituent particles are arranged in a definite, three-dimensional regular geometrical order along all the three axes, in which each particle is depicted as a lattice point. A three-dimensional, regular arrangement of lattice points in space or in a crystal is called a crystal lattice or space lattice.
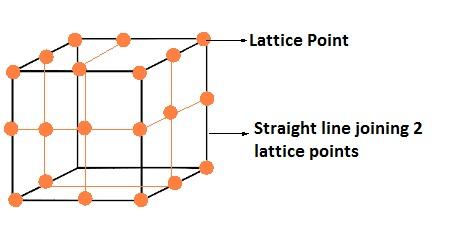
Crystal lattice have following characteristics
- Each point in lattice is called lattice site or lattice point.
- Each lattice point represents one constituent particle i.e. atom, ion or molecule.
- We join lattice points by straight lines to show geometry of the lattice.
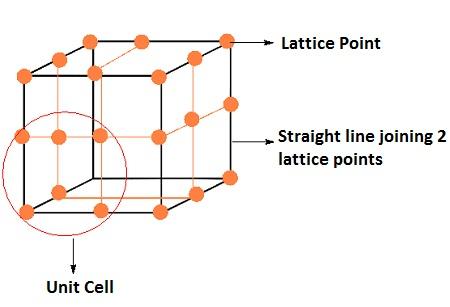
(ii) UNIT CELL
The smallest repeating unit in space lattice which when repeated over and over again in different directions produce complete crystal lattice. There are two important parameters of a unit cell.
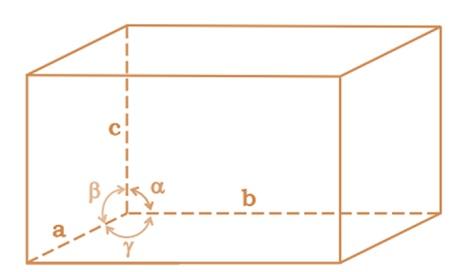
Edge length or Axial Distance: Lengths or dimensions along the three edges a, b and c which may or may not be mutually perpendicular.
Interaxial Angle: Angles α, β and γ between pair of edges are interaxial angle.
- α : between axis B and C
- β : between axis A and C
- γ : between axis A and B
`
Distinction Between Crystalline and Amorphous Solids
| S.No | Crystalline solid | Amorphous solids |
| 1 | These have definite and regular arrangement of the constituent particles in space. | These doesn’t have any regular arrangement of the constituent particles in space. |
| 2 | These are true solids. | Theseare super cooled liquids or pseudo soilds. |
| 3 | These have long order arrangement of the particles. | These have short order arrangement of particle. |
| 4 | These are anisotropic in nature, i.e., their physical properties are different in different directions. | These are isotropic in nature i.e., their physical properties are same in all the directions. |
| 5 | They have sharp melting points. | They melt over a certain range of temperature. |
| 6 | They undergo a clean cleavage when cut. | They undergo irregular cleavage when cut. |
Three-Dimensional Packing
- To understand the packing of constituent particles in a crystal, particles are assumed to be hard spheres of identical size.
- The packing of these hard spheres takes place in such a way that they cover maximum available space and minimum space should be left behind.
- Because of this, the crystal has maximum density. This type of packing is known as close packing.
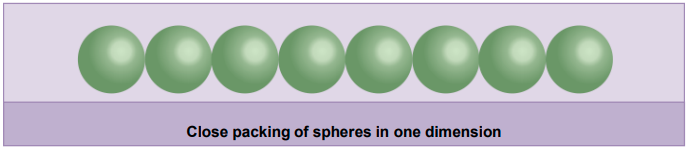
Close Packing in One Dimension
- Spheres are arranged in such a way that they should touch each other in the row.
- In this arrangement, each sphere touches two neighbouring spheres. Hence, the coordination number is 2 in this arrangement.
Close Packing in Two Dimensions
- When the rows of one dimension packing are stacked over each other, a two-dimensional close pack structure is formed.
- This stacking is done in two ways:
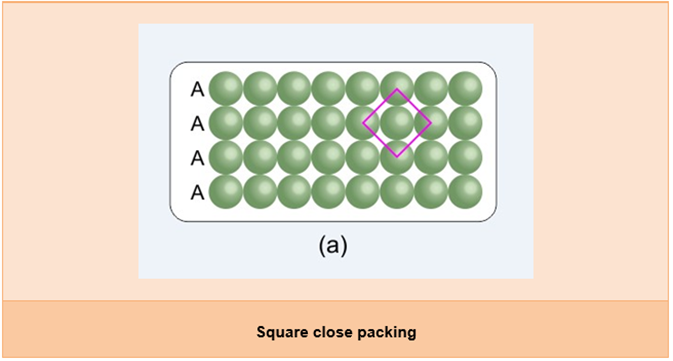
- Square close packing in two dimensions:
- The spheres in the second row are arranged in such a way that they touch the spheres of the first row and are exactly above the first row.
- If the first row is called the ‘A’ type row, then the second row will also be the ‘A’ type as both rows are identical.
- These arrangements are continuous; hence, we can call this arrangement as the AAA type arrangement.
- In this arrangement, each sphere touches four other spheres; hence, the coordination number is four.
- Also, if we join the centres of four spheres touching one particular sphere, then it forms a square; hence, it is called square close packing in two dimensions.
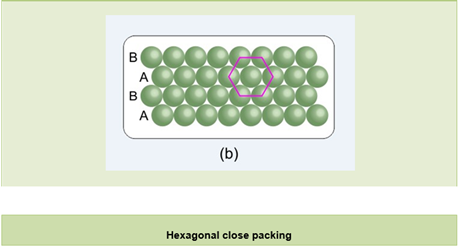
- Hexagonal close packing in two dimensions:
- Spheres in the second row are placed in depressions of the first row. This gives two rows.
- Hence, if we call the first row ‘A’ type, then the second row will be the ‘B’ type.
- If a third row is placed in the depression of the second row, then it will be identical to the first row, i.e. the ‘A’ type.
- The fourth row will be the ‘B’ type and so on. Hence, this arrangement is called the ABAB type of arrangement.
- In this arrangement, each sphere touches six neighbouring spheres; hence, the coordination number is six.
- Also, if we join the centres of the spheres which touch one particular sphere, then it will give a hexagonal structure; therefore, it is known as hexagonal close packing in two dimensions.
- This type of packing is more effective because maximum space is covered by particles and the space is minimum.
Close Packing in Three Dimensions
- Three-dimensional packing can be obtained by square close packing and hexagonal close packing.
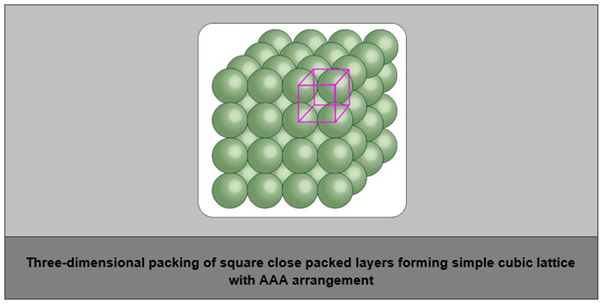
- Three-dimensional close packing from two-dimensional square close packing:
- The second layer and all further layers are arranged in such a way that they are horizontal and vertically aligned with each other.
- Hence, if we call the first layer as the ‘A’ type, then the lattice will be the AAA type.
- This will give simple cubic lattice, and its unit cell will be a primitive cubic unit cell.
- Three-dimensional packing from two-dimensional hexagonal close packing:
- We know that more effective packing is given by hexagonal close packing.
- Assume three-dimensional packing with a hexagonal close packed system. The base layer is called ‘A’ and the voids between the spheres are named ‘a’ and ‘b’ alternately.
- Both ‘a’ and ‘b’ voids are triangular in shape. The only difference is that the apices of voids ‘a’ point downwards and those of ‘b’ point upwards.
- The second layer is placed in such a way that its spheres find place in the ‘a’ voids of the first layer. The ‘b’ voids are left unoccupied because no spheres can be placed in them.
- There are two new types of voids in the second layer—‘c’ and ‘d’ The voids ‘c’ lie above the spheres of the first layer and the voids ‘d’ lie on the voids of the first layer.
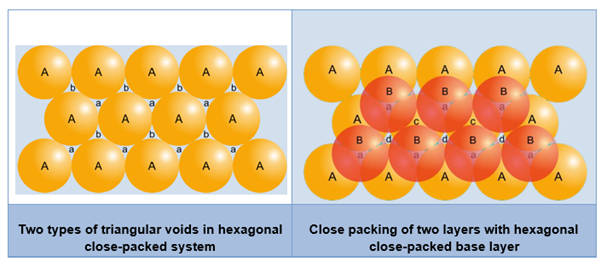
- A simple triangular void ‘c’ which is surrounded by four spheres is called a tetrahedral void. The double triangular void (like ‘d’) which is surrounded by six spheres is called an octahedral void.
- The voids or holes in the crystals are also called interstices.
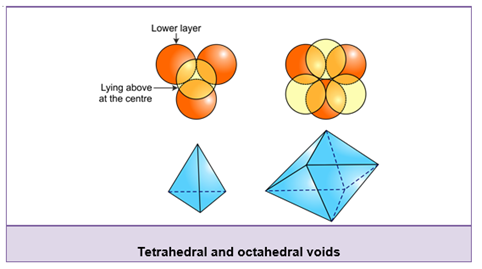
- There are two ways of building the third layer.
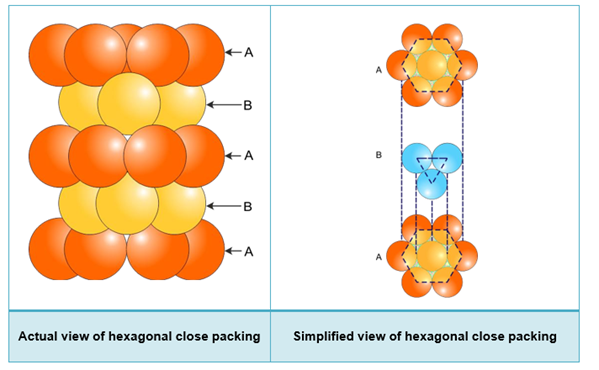
hcp structure:
- When the third layer is placed on the second layer in such a way that the spheres cover the tetrahedral voids, three-dimensional closed packing is obtained.
- Consider the first layer as ‘A’ and the second layer as ‘B’, the arrangement is of the ABAB type or hexagonal closed packing.
- Molybdenum, magnesium and beryllium crystallise in the hcp structure.
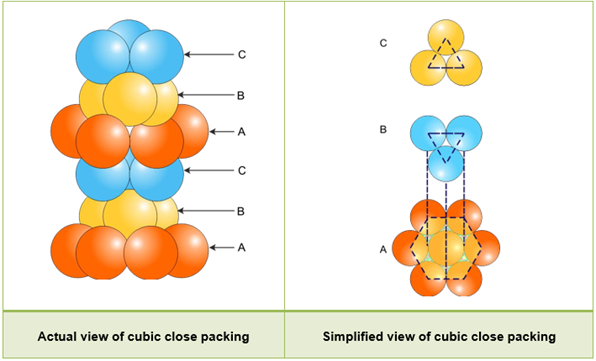
ccp structure:
- When the third layer is placed on the second layer in such a way that the spheres cover the octahedral voids, two layers ‘A’ and ‘B’ are formed. Assume that the new layer be ‘C’.
- On continuing, a packing is obtained where the spheres in every fourth layer will be vertically aligned. This pattern is called the ABCABC pattern or cubic close packing.
- It is the same as face-centred cubic close packing.
- Iron, nickel, copper, silver, gold and aluminium crystallise in the ccp structure.
Packing Efficiency
In all the type of packings there is always some free space in the form of voids or vacant spaces. Packing efficiency is the percentage of total space filled by the particles.
Packing efficiency = Volume of total lattice points / Total volume of unit cell
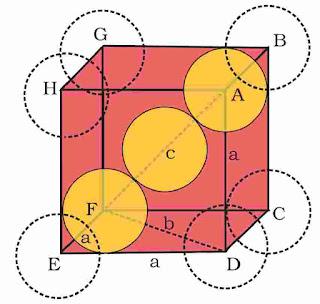
(i). Packing efficiency in fcc or ccp structures
suppose edge length of unit cell = a
And radius of sphere = r
As there are total 4 lattice points per unit cell.
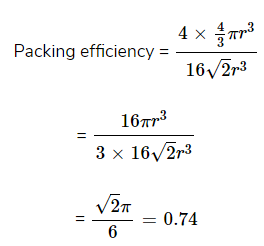
Hence, % Packing efficiency = 74%
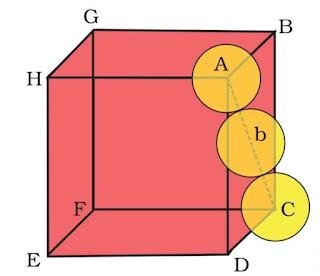
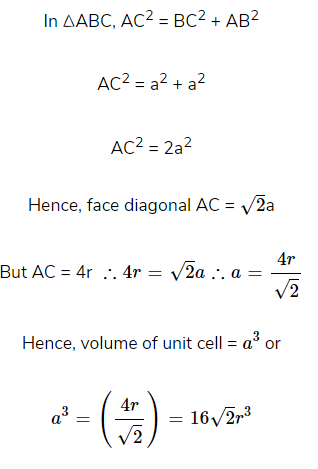
(ii). Packing Efficiency in bcc structures
Suppose the edge length = a
Radius of each sphere = r
Number of lattice points per unit cell = 2
In ΔABC, AC2 = BC2 + AB2 = a2 + a2 = 2a2
Similarly in ΔACD,
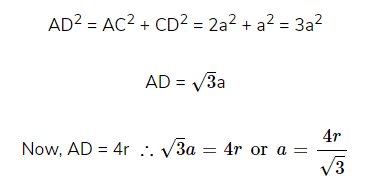
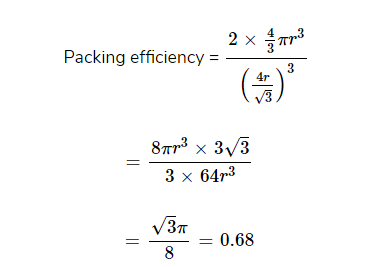
Hence, % of Packing efficiency = 68%
(iii). Packing Efficiency in Simple Cubic Unit Cell
Let the edge length of unit cell = a
Radius of sphere = r
As two spheres touch each other at an edge
∴ a = 2r
Number of lattice points per unit cell = 1
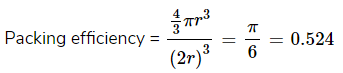
Hence, % Packing efficiency = 52.4%
Coordination Number
- In both hcp and ccp structures, a sphere is in contact with six other spheres in its own layer.
- It also directly touches three spheres above and three spheres below. Thus, the sphere has 12 close neighbours. Hence, it is said to have coordination number 12.
Coordination number: Number of closest neighbours of any constituent particles in the crystal lattice
- The common coordination numbers in different types of crystals are 4, 6, 8 and 12.
- In crystals with directional bonds, the coordination number is lower than that of crystals with a non- directional bond.
- In addition to the above two types, there is another type called body-centred cubic close packing (bcc) in which the space occupied is 68%.
- The coordination number of each atom in the bcc structure is 8.
Point defects:
Point defects explain about the imperfections of solids along with the types of point defects. Crystalline solids are formed by joining many small crystals. Different types of defects are found in crystals after the process of crystallization.
Point defects are accounted for when the crystallization process occurs at a very fast rate. These defects mainly happen due to deviation in the arrangement of constituting particles. In a crystalline solid, when the ideal arrangement of solids is distorted around a point/ atom it is called a point defect.
Defects or Imperfections in crystalline solid can be divided into four groups namely line defects, point defects, volume defects and surface defects. Historically, crystal point defects were first regarded in ionic crystals, not in metal crystals that were much simpler.
There are 3 types of point defects:
- Stoichiometric defect
- Frenkel defect
- Schottky defect
1. Stoichiometric Defect:
In this kind of point defect, the ratio of positive and negative ions (Stoichiometric) and electrical neutrality of a solid is not disturbed. Sometimes it is also known as intrinsic or thermodynamic defects.
Fundamentally, they are of two types:
Vacancy defect: When an atom is not present at their lattice sites, then that lattice site is vacant and it creates a vacancy defect. Due to this, the density of a substance decreases.
Interstitial defect: It is a defect in which an atom or molecule occupies the intermolecular spaces in crystals. In this defect, the density of the substance increases.
A non-ionic compound mainly shows vacancy and interstitial defects. An ionic compound shows the same in Frenkel and Schottky defect.
2. Frenkel Defect:
In ionic solids generally, the smaller ion (cation) moves out of its place and occupies an intermolecular space. In this case, a vacancy defect is created on its original position and the interstitial defect is experienced at its new position.
It is also known as dislocation defect, The density of a substance remains unchanged, It happens when there is a huge difference in the size of anions and cations.
Example: ZnS and AgCl.
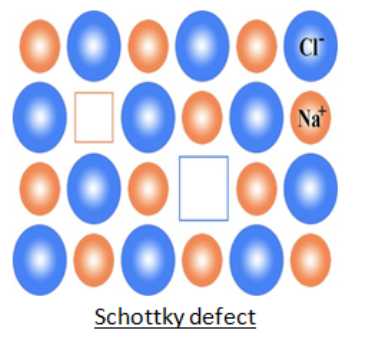
3. Schottky Defect
This kind of vacancy defects is found in Ionic Solids. But in ionic compounds, we need to balance the electrical neutrality of the compound so an equal number of anions and cations will be missing from the compound. It reduces the density of the substance. In this, the size of cations and anions are of almost the same.
Impurity Defect: Let’s understand the impurity defect by an example. If molten NaCl is crystallized with SrCl2 compound then the Sr2+ ions replace two Na+ ions and occupy the place of one Na+ In this way the lattice site of one Na+ is vacant and it creates an impurity defect.
Non-Stoichiometric Defect: In this defect, the cations and anions ratio is disturbed either because of adding or removing of ions.
Types of Non-Stoichiometric Defects:
Metal deficiency defect: In this, the solids have less number of metals relative to the described Stoichiometric proportion.
Metal excess defect:
There are two types of metal excess defect: Metal excess defect due to anionic vacancies: This occurs due to the absence of anions from its original lattice site in crystals. Therefore, instead of anions, electrons occupy their position
Metal excess defect due to the presence of extra cations at interstitial sites: Here, on heating the compound, it releases extra cations. These cations occupy the interstitial sites in crystals and the same number of electrons goes to neighbouring interstitial sites.
Magnetic Properties:
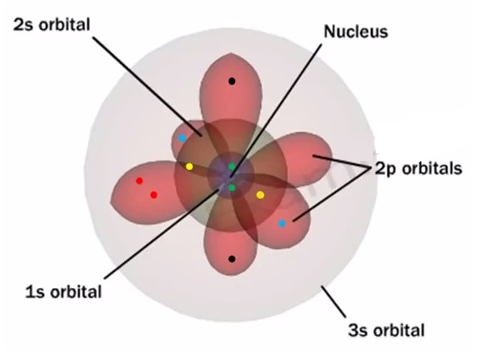
- Every substance possesses magnetic properties originated from the electrons present in them.
- Each electron in an atom behaves like a tiny magnet, The magnetic moment of these substances originates from two types of motions its orbital motion around the nucleus and its spin around its own axis.
- Electron being a charged particle undergoes these motions and can be considered as a small loop of current possessing a magnetic moment, Therefore, each electron has a permanent spin and an orbital magnetic moment associated with it.
- Magnitude of this magnetic moment is very small and is measured in the unit called Bohr magneton, μ B and is equal to 9.27 × 10 – 24A m2.
On the basis of their magnetic properties, substances can be classified into five categories:
- Paramagnetic: Paramagnetic materials are materials that tend to get weakly magnetized in the direction of the magnetizing field when placed in a magnetic field. Paramagnetic materials have a permanent dipole moment or permanent magnetic moment. However, if we remove the applied field the materials tend to lose their magnetism. This is because thermal motion randomizes the spin orientations of the electrons.
- Diamagnetic: The spin motion of electrons and their interaction with one another is what is responsible for the origin of magnetism. The best way to introduce different types of magnetic materials is by describing how materials respond to magnetism. It may come as a surprise to you, but all matter is magnetic. The only difference is that some materials are more magnetic than others. The level of interactions between the magnetic moments is what distinguishes them. In a few materials, there is no collective interaction of atomic magnetic moment while other materials exhibit strong atomic magnetic moment interaction.
- Ferromagnetic: There are various kinds of magnetism, out of which ferromagnetism is the strongest type. Ferromagnetic materials are those materials which exhibit a spontaneous net magnetization at the atomic level, even in the absence of an external magnetic field.
When placed in an external magnetic field, ferromagnetic materials are strongly magnetized in the direction of the field. Ferromagnetic materials are strongly attracted to a magnet. These materials will retain their magnetization for some time even after the external magnetizing field is removed. This property is called hysteresis.
- Anti-ferro magnetic: The materials that exhibit the anti-ferro magnetism are known as antiferromagnetic material. When these materials are kept in the presence of the strong magnetic field, they get magnetized weakly in the direction of the magnetic field. This is known as anti-ferro magnetism.
The antiferromagnetic materials are commonly found among the transition metal compounds. Hematite, chromium, alloys of iron manganese and oxides of nickel are the examples of antiferromagnetic material
- Ferrimagnetic: Ferrimagnetic materials definition is, in which the magnetic dipoles of the atoms on various subset are opposed, as in anti-ferro magnetism, but the opposing moments are unequal in ferrimagnetic materials, leaving a random net magnetization. Crystal ferrimagnetic materials, including antiferromagnetic materials, have populations of atoms with contrasting magnetic moments. Since the magnitudes of these moments are unequal in ferrimagnet compounds, a random magnetization exists. A mixture of dipole-dipole interactions and exchange interactions arising from the Pauli exclusion theory induce magnetization of ferrimagnetic materials. The key distinction is that in a ferrimagnetic substance, the unit cell contains various groups of atoms.
Semiconductor
Semiconductors are substances with properties somewhere between them. ICs (integrated circuits) and electronic discrete components such as diodes and transistors are made of semiconductors. Common elemental semiconductors are silicon and germanium. Silicon is well-known of these. Silicon forms most of ICs.
Conduction of Electricity in Semiconductors In semiconductors, the gap between the valence band and conduction band is small.
This enables some electrons to jump to conduction band and exhibit their conductivity.
Electrical conductivity of semiconductors increases with increase in temperature, since more electrons can jump to the conduction band due to small gap between the valence band and conduction band.
Silicon and germanium exhibit this behavior and are called intrinsic semiconductors. The conductivity of these intrinsic semiconductors is too low to be practically used.
Semiconductor materials
Solid-state materials are commonly grouped into three classes: insulators, semiconductors, and conductors. (At low temperatures some conductors, semiconductors, and insulators may become superconductors.) The figure shows the conductivities σ (and the corresponding resistivities ρ = 1/σ) that are associated with some important materials in each of the three classes. Insulators, such as fused quartz and glass, have very low conductivities, on the order of 10−18 to 10−10 siemens per centimeter; and conductors, such as aluminum, have high conductivities, typically from 104 to 106 siemens per centimeter. The conductivities of semiconductors are between these extremes and are generally sensitive to temperature, illumination, magnetic fields, and minute amounts of impurity atoms. For example, the addition of about 10 atoms of boron (known as a dopant) per million atoms of silicon can increase its electrical conductivity a thousand fold
n-type Semiconductor:
An n-type semiconductor is an intrinsic semiconductor doped with phosphorus (P), arsenic (As), or antimony (Sb) as an impurity. Silicon of Group IV has four valence electrons and phosphorus of Group V has five valence electrons. If a small amount of phosphorus is added to a pure silicon crystal, one of the valence electrons of phosphorus becomes free to move around (free electron*) as a surplus electron. When this free electron is attracted to the “+” electrode and moves, current flows.
p-type Semiconductor:
A p-type semiconductor is an intrinsic semiconductor doped with boron (B) or indium (In). Silicon of Group IV has four valence electrons and boron of Group III has three valence electrons. If a small amount of boron is doped to a single crystal of silicon, valence electrons will be insufficient at one position to bond silicon and boron, resulting in holes* that lack electrons. When a voltage is applied in this state, the neighboring electrons move to the hole, so that the place where an electron was present becomes a new hole, and the holes appear to move to the “–” electrode in sequence.
Applications of n-type and p-type semiconductors:
n-type and p-type semiconductors finds a great use in manufacturing electronic components.
Diode is a combination of n-type and p-type semiconductors extensively used as a rectifier.
Transistors are manufactured by keeping a layer of one type of semiconductor between two layers of another type of semiconductor.
npn and pnp type of transistors are used to detect or amplify radio or audio signals.
The solar cell is an efficient photo-diode used for conversion of light energy into electrical energy.
Gallium arsenide (GaAs) semiconductors have very fast response and have transformed the design of semiconductor devices.
Transition metal oxides show marked differences in electrical properties.
TiO, CrO2 and ReO3 behave like metals.
Rhenium oxide, ReO3 resembles metallic copper in terms of its conductivity and appearance.
Certain other oxides like VO, VO2, VO3 and TiO3 exhibit metallic or insulating properties depending on temperature.
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
