NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 2, “Solutions,” is a comprehensive study material package that provides students with all the necessary resources to understand the concepts of solutions.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including the types of solutions, solubility, concentration, colligative properties, and the vapour pressure of solutions. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the definition of solutions to the factors affecting the solubility of solids and gases. It also includes questions on the different types of concentration units and colligative properties, such as boiling point elevation and freezing point depression.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the definition of solutions, the types of solutions, and the factors affecting the solubility of solids and gases.
NCERT Solution Class 12 Chemistry Chapter 2 Solutions with Mind Map PDF Download
Introduction:
In this chapter, we will discuss about liquid solutions and their formation. This will be followed by studying the properties of solutions, like vapour pressure and colligative properties. We will begin with types of solutions and expressions for concentration of solutions in different units.
Thereafter, we will state and explain Henry’s law and Raoult’s law, distinguish between ideal and non-ideal solution and deviation of real solutions from Raoult’s law. We will also discuss abnormal colligative properties alongwith association and dissociation of solute.
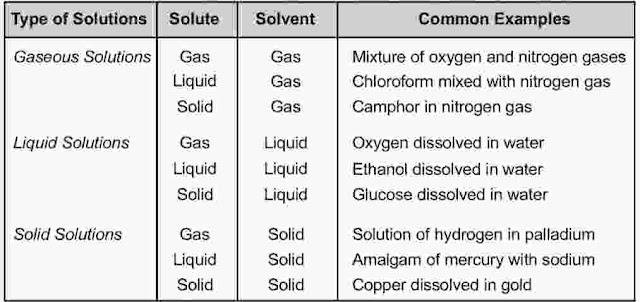
Types of Solutions
All the three states of matter (solid, liquid and gas) may behave either as solvent or solute. When a solution is composed of only two chemical substances, it is termed as binary solution. Depending upon the state of solute or solvent, binary solutions can be classified as
Some Important Definitions
- Mixture – When two or more chemically non-reacting substances are mixed, they form mixture.
- Heterogeneous Mixture – It consists of distinct phases, and the observed properties are just the sum of the properties of individual phases.
- Homogeneous Mixture – It consists of a single phase which has properties that may differ from one of the individual components.
- Solution – The homogeneous mixture of two or more components such that at least one component is a liquid is called solution.
- Solvent – It is the constituent of solution which has same physical state as that of solution and generally present in greater amount than all the other components.
- Solute – The component of a solution other than solvent is called solute, may or may not have same physical state as that of solution. Generally it is in smaller amount.
Example – In a sugar syrup (liquid solution) containing 60% sugar (solid) and 40% water (liquid), water is termed as a solvent, due to the same physical state as that of the solution.
Expressing the Strength of Solution
For a given solution the amount of solute dissolved per unit volume of solution is called concentration of solute. Strength of solution is the amount of solute in grams dissolved in one litre of solution. It is generally expressed in g/litre.
Other methods of expressing the strength of solution are:
- Mass percentage –
Mass % of solute =Mass of soluteTotal mass of solution×100
Mass % of solvent =Mass of solventTotal mass of solution×100
- Volume percentage –
Volume % of solute =Volume of soluteTotal volume of solution×100
Volume % of solvent =Volume of solventTotal volume of solution×100
- Molality (m) – It is no. of moles of solute dissolved in 1 kg of the solvent.
m =(Number of moles of solute)(Mass of solvent {in kg})
- Molarity (M) – It is no. of moles of solute dissolved in 1 litre of solution.
M =(Number of moles of solute)(Mass of soution {in litre})
- Normality (N) – It is no. of gram-equivalents of solute dissolved in 1 litre of solution
N =(Number of gram equivalents of solute)(Volume of solution {in litre)
- Formality – Ionic solutes do not exist in the form of molecules. These molecular mass is expressed as Gram-formula mass. Molarity for ionic compounds is actually called as formality.
- Mole fraction –
Mole fraction of solute =(Number of moles of solute)(Total moles of solution)
Mole fraction of solvent =(Number of moles of solvent)(Total moles of solution)
For a binary solution,
mole fraction of solute + mole fraction of solvent = 1.
- Parts per million (ppm) –
It is defined in two ways
ppm = mass fraction × 106
ppm = mole fraction × 106
Solubility
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specified temperature. It depends upon the nature of solute and solvent as well as temperature and pressure. Let us consider the effect of these factors in solution of a solid or a gas in a liquid.
- Solubility of Solid in Liquid
A solute dissolves in a solvent if the intermolecular interactions are similar in them, i.e., like dissolves like. Polar solute dissolves in polar solvent and non-polar solute in non-polar solvent. For e.g., sodium chloride and sugar dissolves readily in water and napthalene and anthracene dissolves readily in benzene.
Solute + Solvent ⟶ Solution
- Dissolution: When a solid solute is added to the solvent, some solute dissolves and its concentration increases in solution. This process is called dissolution.
- Crystallization: Some solute particles collide with solute particles in solution and get separated out. This process is called crystallization.
- Saturated solution: Such a solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution.
- Unsaturated solution: An unsaturated solution is one in which more solute can be dissolved at the same temperature.
- Effect of temperature: In general, if in a nearly saturated solution, the dissolution process is endothermic, the solubility should increase with rise in temperature, if it is exothermic, the solubility should decrease with rise in temperature.
- Effect of pressure: Solids and liquids are highly incompressible, so pressure does not have any significant effect on solubility of solids and liquids.
- Supersaturated solution: When more solute can be dissolved at higher temperature in a saturated solution, then the solution becomes supersaturated.
- Solubility of Gas in Liquid
All gases are soluble in water as well as in other liquids to a greater or lesser extent. The solubility of a gas in liquid depends upon the following factors Nature of the gas, Nature of solvent, Temperature and Pressure.
Generally, the gases which can be easily liquified are more soluble in common solvents. For e.g., CO2 is more soluble than hydrogen or oxygen in water. The gases which are capable of forming ions in aqueous solutions are much more soluble in water than other solvents. For e.g., HCl and NH3 are highly soluble in water but not in organic solvents (like benzene) in which they do not ionize.
- Effect of temperature: Solubility of most of the gases in liquids decreases with rise in temperature. In dissolution of a gas in liquid, heat is evolved and thus this is an exothermic process. The dissolution process involves dynamic equilibrium and thus follows Le Chatelier’s principle. As dissolution is exothermic the solubility of gas should decrease with rise in temperature.
- Effect of pressure: Henry’s law: At constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
p = KH x,
KH = Henry’s law constant.
Applications of Henry’s law
- In manufacture of soft drinks and soda water, CO2 is passed at high pressure to increase its solubility.
- To minimise the painful effects accompanying the decompression of deep sea divers. O2 diluted with less soluble. He gas is used as breathing gas.
- At high altitudes, the partial pressure of O2 is less then that at the ground level. This leads to low concentrations of O2 in the blood of climbers which causes ‘anoxia’.
Vapour Pressure of Solution
It is the pressure exerted by vapour on the surface layer of liquid at equilibrium between vapour and liquid.
Factors affecting Vapour Pressure
- Nature of liquid – Liquid with higher intermolecular attraction forces form less amount of vapour and hence lower vapour pressure and vice-versa.
- Temperature – Vapour pressure increases with temperature of liquid. This is because, as temperature increases, kinetic energy of the molecules increases, hence, more molecules leave the surface of the liquid and come into vapour phase.
Raoult’s Law
According to Raoult’s law, for a solution of volatile liquids, the relative lowering of vapour pressure of solution is directly proportional to its mole fraction of dissolved solvent in solute.
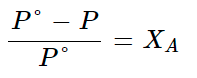
Ideal and Non-Ideal Solution
The binary liquid-liquid solution may be classified into two types :
(1) Ideal solutions
(2) Non-ideal solutions
1) Ideal Solutions
An ideal solution may be defined as the solution which obeys Raoult’s law over the entire range of concentration.
a) Such solutions are formed by mixing two components which are identical in molecular size, in structure and have almost identical intermolecular forces.
b)The intermolecular interactions between the components (A – B attractions) are of same magnitude as the intermolecular interactions in pure components (A- A and B-B attractions).
According to Raoult’s law, the partial vapour pressure of two components of the solution may be given as :
pA = pA°xA
pB = pB°xB
Total pressure p is given by
p= pA +pB
p= pA°xA + pB°xB
The ideal solutions have also the following characteristics:
1) Heat change on mixing is zero: Since there is no change in magnitude of the attractive forces in the two components present, the heat change on mixing i.e. ΔmixingH in such solutions must be zero.
2) Volume change on mixing is zero: In ideal solutions, the volume of the solution is the sum of the volumes of the components before mixing i.e. there is no change in volume on mixing or Δmixing V is zero.
The solutions generally tends to become ideal when they are dilute.
The characteristics of an ideal solution may be summed up as follows
(i) It must obey Raoult’s law.
(ii) Δmixing H should be zero.
(iii) Δmixing V should be zero.
Example of Ideal Solutions
(i) Benzene and toluene
(ii) n-hexane and n-heptane
(iii) Bromoethane and iodoethane
(iv) Chlorobenzene and bromobenene
Solutions which obey Raoult’s law are called ideal liquid solution.
Composition in Vapour Phase
The composition of vapour phase in equilibrium with the solution is determined by the partial pressure of the components. If y1and y2 are the mole fraction of the two components 1 and 2 respectively in the vapour phase, then according to Dalton’s law of partial pressures:
Partial pressure of a component =Mole fraction of the component x Total pressure
p1= y1 p
p2 =y2 p
In general, Pi = yi p total
Mole fraction of component 1 in vapour phase y1 = p1/p
Mole fraction of component 2 in vapour phase, y2 = p2 /p
Mole fraction of a component in vapour phase =
Partial vapour pressure of component / Total Vapour Pressure
2) Non-Ideal Solutions
The solutions which do not obey Raoult’s law over the entire range of concentration are called non-ideal solutions. Therefore, for such solutions
pA ≠ pA° xA
pB ≠ pB° xB
The vapour pressure of such solutions is either higher or lower than that predicted by Raoult’s law.
In non-ideal solutions, there is a noticeable change in volume and heat energy when the two components are mixed.
Most of the solutions are non-ideal because they deviate from ideal behaviour to more or less extent.
Thus, for non-ideal solutions,
a) none of the components obey Raoult’s law over the entire composition range, i.e.
pA ≠ pA° xA and pB ≠ pB° xB
b) (i) Δmixing H is not equal to zero
(ii) Δmixing V is not equal to zero
| Ideal solution | Non-Ideal solution |
| The interactions between the components are similar to those in the pure components. | The interaction between the components are different from those of the pure components. |
| There is no enthalpy change on mixing.ΔmixingH =0 | There is no enthalpy change on mixing.ΔmixingH ≠ 0 |
| There is no volume change on mixing.Δmixing V =0 | There is no volume change on mixing.Δmixing V ≠ 0 |
| Each component obeys Raoult’s law at all temperatures and concentrations,pA = pA°xA and pB = pB°xB | Their components do not obey Raoult’s law. They show positive and negative deviations from Raoult’s law.pA ≠ pA° xA and pB ≠ pB° xB |
Types of Non-ideal Solutions
Non-ideal solutions show positive and negative deviations from the ideal behaviour depending upon their nature.
(1) Non-Ideal solutions showing positive deviations from Raoult’s law
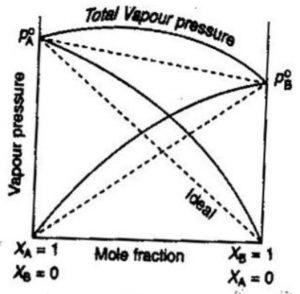
Consider a binary solution of two components A and B.
If the A-B interactions in the solutions are weaker than the A-A and B-B interactions in the two liquids forming the solution, then the escaping tendency of A and B types of molecules from the solution becomes more than from pure liquids. As a result, each component of solution has a partial vapour pressure greater than expected on the basis of Raoult’s law. The total vapour pressure will be greater than corresponding vapour pressure expected in case of ideal solution of the same composition. This type of behaviour of solution is described as positive deviations from Raoult’s law.
pA >pA° xA and
pB >pB° xB
The total vapour pressure,
p= pA+ pB is always greater than (pA° xA + pB° xB )
Examples of solutions showing positive deviations are :
(i) Ethyl alcohol and cyclohexane
(ii) Acetone and carbon disulphide
(iii) Benzene and acetone
(iv) Carbon tetrachloride and choloroform
(v) Acetone and ethyl alcohol
(vi) Ethyl alcohol and water.
Explanation for positive deviations
In ethyl alcohol, the molecules are held together due to hydrogen bonding. When cyclohexane is added to ethyl alcohol, the molecules of cyclohexane tend to occupy the spaces between ethyl alcohol molecules. Consequently, some hydrogen bonds in alcohol molecules break and the attractive forces in alcohol molecules are weakened. The escaping tendency of alcohol and cyclohexane molecules from the solution increases. Consequently, the vapour pressure of the solution is greater than the vapour pressure as expected according to Raoult’s law.
(i) Δmixing H is positive because energy is required to break A-A or B-B attractive forces. Therefore, dissolution process is endothermic.
(ii) Because of the decrease in the magnitude of intermolecular forces in solutions, the molecules will be loosely held and, therefore, there will be increase in volume on mixing. Thus, Δmixing V will be positive.
(iii) Since the dissolution process in endothermic, heating will increase the solubility of such a solution.
(2) Non-Ideal solutions showing negative deviations from Raoult’s law.
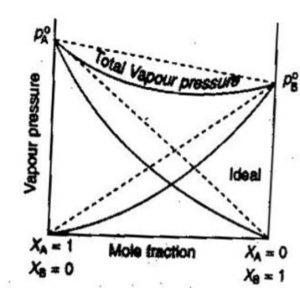
In such solutions, the A-B interactions are stronger than the A-A and B-B interactions present in the two liquids forming the solution. Due to stronger A-B interactions, the escaping tendency of A and B types of molecules from the solution becomes less than from pure liquids. Consequently, each component of the solution has a partial vapour pressure less than expected on the basis of Raoult’s law. As a result , the total vapour pressure becomes less than the corresponding vapour pressure expected in case of ideal solution.
pA < pA° xA and
pB < pB° xB
p= pA+ pB is always less than (pA° xA + pB° xB )
Examples of Negative Deviation
(i) Acetone and chloroform
(ii) Chloroform and diethyl ether
(iii) Chloroform and nitric acid
(iv) Acetone and aniline
(v) Water and nitric acid
(vi) Diethyl ether and chloroform.
Explanation for Negative Deviations
When acetone and chloroform are mixed there are new attractive forces due to intermolecular hydrogen bonding. Thus the attractive forces become stronger and the escaping tendency of each liquid from the solution decreases. Therefore, the vapour pressure of the solution is less than that expected for an ideal solution.
(i) ΔmixingH is negative because energy is released due to increase in attractive forces. Therefore, dissolution process is exothermic and heating the solution will decrease solubility.
(ii)Because of the increase in the magnitude of forces of attraction in solutions, the molecules will be loosely held more tightly. Therefore, there will be decrease in volume on mixing. Thus, ΔmixingV will be negative.
Difference between Ideal and Non-Ideal solution
| Solution having positive deviation | Solution having negative deviation |
| A-B forces are less than A-A and B-B forces. | A-B forces are more than A-A and B-B forces. |
| pA >pA° xA and pB >pB° xB | pA < pA° xA and pB < pB° xB |
| ΔmixingH is positive | ΔmixingH is negative |
| Dissolution is endothermic | Dissolution is exothermic |
| Heating increases solubility | Heating decreases solubility |
| ΔmixingV will be positive. | ΔmixingV will be negative. |
a) Some solution exhibit very large positive deviation from Raoult’s law that there is a maximum in the vapour pressure curve which is above the vapour pressure of either pure components.
For one of the intermediate composition, the total vapour pressure of such a solution will be the highest and the boiling point will be the lowest. At this point, the composition of liquid and vapour phase is same and the liquid mixture boils at constant temperature and remains unchanged in composition. Therefore, this liquid mixture distills over as if it is a pure liquid. Solution acquires the property of boiling at constant temperature and remains unchanged in composition.
The solutions (liquid mixtures) which boil at constant temperature and can distil unchanged in composition are called azeotropes or azeotropic mixtures. Thus, the azeotropes distil over as if it were pure liquids. These types of solutions are called minimum boiling azeotropes.
For example: Ethanol and water form minimum boiling azeotrope. It has maximum in the vapour pressure curve and hence a minimum in the boiling point diagram. In this boiling point diagram, we indicate the composition of the vapour phase by the upper curve and composition of the liquid phase by the lower curve.
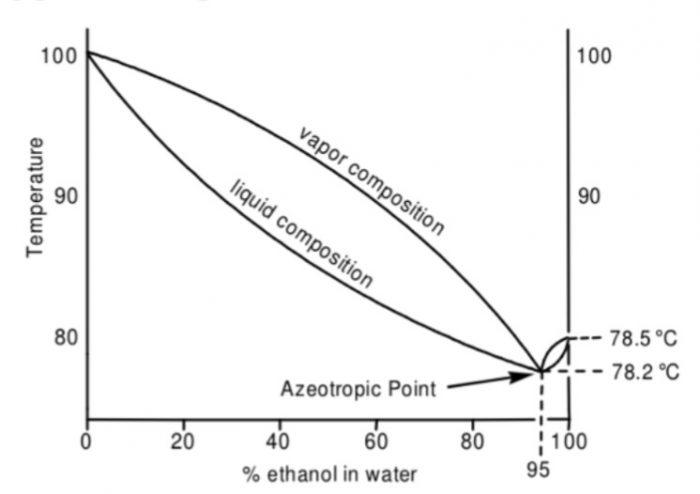
The boiling point diagram shows an azeotropic composition at x(H2O)=0.056 and x(C2H5OH) = 0.944 at a temperature.of 351 K (or 78°C) which is lower than that of pure ethanol 351.5 K (or 78.5°C) and water 378 K (or 100°C).
Fractional distillation of solutions lying on either side of this azeotropic composition is capable of separating them into at best, one pure component and a azeotropic mixture having the minimum boiling point.
b) In the case of solutions showing negative deviations, total vapour pressure becomes less than the corresponding ideal solution of same composition. The boiling points of such solutions are increased.
For one of the intermediate composition, the total vapour pressure will be the least and the boiling point will be the highest. At this composition, the solution also boils at constant temperature without a change in composition. This is also called azeotrope.
The solutions which show negative deviations from Raoult’s law are called maximum boiling azeotropes because they have a composition having maximum boiling point
For example: hydrochloric acid and water form maximum boiling point azeotrope at the composition x(H2O)=0.889 and x (HCl)=0.111 (or 20.2% HCl) which boils at 381.6 K (or 108.6°C) which has higher than that of pure water.
Nitric acid (HNO3) and water also form maximum boiling azeotrope. The azeotrope has the approximate composition 68% nitric acid and 32% water by mass with a boiling point of 393.5 K.
Thus, the azeotropes are liquid mixtures of definite composition, which boil at constant boiling point. These cannot be separated into pure components by fractional distillation.
Colligative Properties
The properties of dilute solution which depends only on number of particles of solute (molecules or ions) present in the solution and not on their nature, are called colligative properties. The important colligative properties are;
- Relative lowering of vapour pressure
- Elevation of boiling point
- Depression in freezing point
- Osmotic pressure
- Relative Lowering of Vapour Pressure
When a non-volatile solute is added to a solvent, its vapour pressure gets lowered. If this pressure is divided by pressure of pure solvent, this is called relative lowering of vapour pressure.
According to Raoult’s law,
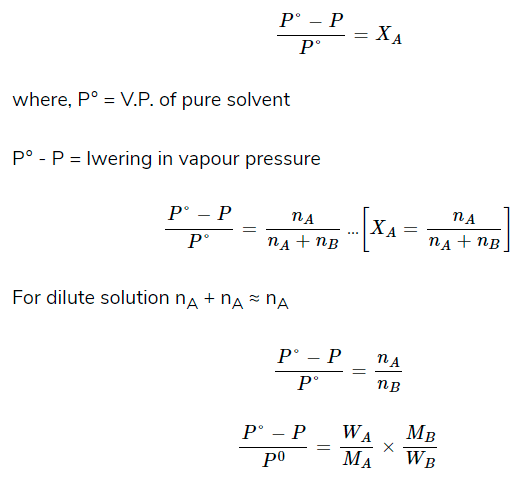
where, WA = weight of solute
WB = weight of solvent
MA = molecular weight of solute
MB = molecular weight of solvent
- Relative Elevation of boiling point
A liquid boils at the temperature at which its vapour pressure is equal to the atmospheric pressure. The boiling point of a solution of non-volatile solute is always higher than that of the boiling point of pure solvent in which the solution is prepared. Similar to lowering of vapour pressure, the elevation of boiling point also depends on the number of solute particles rather than their nature.
Let T° be the boiling point of pure solvent and T be the boiling point of solution. The increase in boiling point ΔTb = T – T° is known as elevation in boiling point.
For dilute solutions, the ΔTb is directly proportional to the molal concentration of the solute in a solution. Thus
ΔTb ∝ m
ΔTb = Kbm
Kb is molal elevation constant (Ebullioscopic constant). The unit of Kb is K kg mol–1.
Substituting the value of molality in above equation, we get
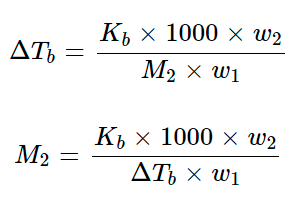
Where, w1 = mass of solvent, w2 = mass of solute and M2 = molar mass of solute
- Depression in freezing point
Freezing point is the temperature at which vapour pressure of liquid phase becomes same as that of solid phase. The decrease in freezing point of a solvent on the addition of a non-volatile solute is known as depression in freezing point.
Let T° be the freezing point of pure solvent and T be the freezing point of solution. The decrease in freezing point ΔTf = T° -T is known as depression in freezing point.
For dilute solutions, the ΔTf is directly proportional to the molal concentration of the solute in a solution. Thus
ΔTf ∝ m
ΔTf = Kf ⋅ m
Here Kf is molal depression constant or cryoscopic constant
Substituting the value of molality in above equation, we get
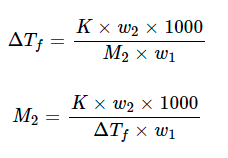
Where, w1 = mass of solvent, w2 = mass of solute and M2 = molar mass of solute
- Osmotic pressure
Osmosis is the spontaneous flow of the solvent molecules from a less concentrated solution (dilute) to a more concentrated solution through a semi-permeable membrane. The driving force of osmosis is called osmotic pressure. Osmotic pressure may be defined as “the minimum excess pressure that has to be applied on the solution to prevent the osmosis”.
Osmotic pressure of a solution ∝ molar concentration of solute in that solution
π ∝ c
π = cRT
where, R = Gas constant = 0.0821 lit atm K-1 mole-1
T = Temperature
c = Molar concentration
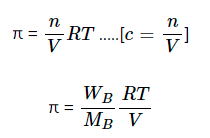
WB = wt. of solute
MB = Molar mass of solute
van’t Hoff Factor
To calculate the extent of association or dissociation, van’t Hoff in 1886 introduced a factor ‘i’ called van’t Hoff factor. van’t Hoff factor ‘i’ is defined as ratio of the experimental value of colligative property to the calculated value of colligative property.
i.e., iExperiment ecolligatival propertiesCalculated ecolligativ properties
Abnormal Molar Masses
Abnormal Molecular Masses
The accurate values of molar masses can be obtained only if the following two conditions are met.
(i) The solutions should be dilute
The solutions used for measuring colligative properties must not be too concentrated. In the concentrated solutions, the particles begin to interact with each other as well as with the solvent. As a result, the vapour pressure and therefore, other colligative properties depend upon the nature of the solute and not just on the number of solute particles.
(ii) The solute must not dissociate or associate in solution
The equations derived for measuring the colligative properties are for non-electrolyte solutes which do not undergo any dissociation or association in the solution.
Discrepancies in determination of molar mass arise when the solute dissociate or associate on dissolving in a solvent. This is because due to the association or the dissociation of the solute molecules in the solution, the number of molecules undergo a change.
The discrepancy in molar mass is called abnormal molar mass. The abnormal molar masses is due to association or dissociation of solute particles.
Association of Solute Particles
In certain solvents, generally non-polar, the solute molecules undergo association i.e. two, three or even more molecules exist in combination with each other to form bigger molecules. For example: suppose n simple molecules combine to form an associated molecule as:
nA ⇔ An
Therefore, the total number of molecules in solution become less than the number of molecules of the substance added and, therefore, colligative properties will be lower. Since the colligative properties are inversely proportional to the molar mass of the solute, the molar masses in such cases will be greater than the theoretical values.
For example: In benzene solvent, both ethanoic acid (acetic acid) and benzoic acid exist as dimers as:
2CH3COOH ⇔ (CH3COOH)2
2C6H5COOH ⇔ (C6H5COOH)2
The molar masses of ethanoic acid and benzoic acid have been found to be nearly 120 and 244 which are about double than their normal values of 60 and 122. The association of solute molecules in a solution is generally due to the hydrogen bonding between these molecules.
For example: benzoic acid and ethanoic acid exist as dimers due to the formation of hydrogen bonds. Due to association of ethanoic acid or benzoic acid, the ΔTf or ΔTb value will be about half of the normal value. Therefore, the molar mass calculated on the basis of ΔT will be about twice the expected value.
Dissociation of Solute Molecules
Molecules of certain substances (acids, bases and salts) dissociate or ionise in a solvent to give two or more particles.
For example: AB dissociates to give double number of particles as:
AB ⇔ A+ + B–
The total number of particles increases in solution and, therefore, the colligative properties of such solutions will be large. Since colligative properties are inversely proportional to molar mass, the observed molar mass will be less than the theoretical value.
For example, KCl dissociates to give K+ and Cl– ions.
KCl ⇔ K+ + Cl–
If we dissolve 1 mol of KCl (74.5 g) in water, we expect 1 mol of K+ and 1 mol of Cl– ions to be produced in the solution. Therefore, there would be 2 mol of particles in the solution instead of 1 mol. Consequently, the colligative properties would also be about double than expected.
For example: if we ignore interionic attraction, 1 mol of KCl in 1 kg of water would be expected to increase the boiling point by 2 X 0.52 K (Kb= 0.52 Km-1) = 1.04 K.
Van’t Hoff Factor
In 1886, Van’t Hoff introduced a factor called Van’t Hoff factor, i , to express the extent of association or dissociation of solutes in solution. It is the ratio of the normal and observed molar masses (or abnormal molar mass) of the solute, i.e.
i=Normal molar massObserved molar mass
In case of association, observed molar mass being more than the normal, the factor i has a value less than 1.
But in case of dissociation, the Van’t Hoff factor is more than 1 because the observed molar mass has a lesser value.
In case of solutes which do not undergo any association or dissociation in a solvent, the Van’t Hoff factor i will be equal to 1 because the observed and normal molar masses will be same.
Since the molar masses are inversely proportional to the colligative property. Van’t Hoff factor may also be expressed as:
i=Observed colligative propertyNormal collegative property
Relative lowering in vapour pressure = i xsolute = Δp / p°
Elevation in boiling point , Δ Tb =i Kb m
Depression in freezing point = Δ Tf = i Kf m
Osmotic pressure π = i cRT
Van’t Hoff Factor and Extent of Dissociation or Association in an Electrolytic Solution
Van’t Hoff factor can be used to calculate the extent of dissociation or association in terms of degree of dissociation or association of a substance in solution.
(i) Degree of dissociation: It is defined as the fraction of total substance that undergoes dissociation into ions, i.e.
Degree of dissociation = No. of moles of the substance dissociated / Total number of moles of the substance taken
Suppose a molecule of an electrolyte gives n ions after dissociation. Then if we start with 1 mole of the solute, and α is the degree of dissociation, then at equilibrium:
Number of moles of solute left undissociated =1-α
Number of moles of ions formed = nα
Total number of moles of particles 1-α+ nα
Van’t Hoff factor, i =
Observed number of moles of solute / Normal number of moles of solute
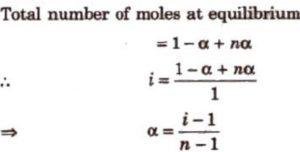
Knowing the value of i from observed molar mass and normal molar mass, degree of dissociation, α can be calculated.
For the electrolytes of the type AB, such as KCl, NaCl, etc,.the number of particles in solution i.e. n = 2
For the electrolytes of the type AB, like CaCl2, Ba(NO3)2 etc, the value of n=2, so that
α = ( i-1 ) /2
(ii) Degree of association: It is defined as the fraction of total number of molecules which combine to form associated molecules, i.e.
Degree of association=No. of moles of the substance associatedTotal number of moles of substance taken
For example: suppose n simple molecules of the solute associate to form the associated molecule A.
nA= An
If we start with 1 mole of A and α is the degree of association, the concentration of the species after association is
[An] = α/n
[A] =1-α
Total number of moles after association = 1-α+ α/n
Thus, the colligative properties will correspond to (1-α+ α/n) mole particles rather than one mole of particles.
Van’t Hoff factor, i , is : Observed number of moles of solute / Normal number of moles of solute
= 1-α + α/n / 1
= 1+ α (1/n -1)
α =( i-1 ) /( 1/n -1)
I=Normal molar mass Observed molar mass
Knowing n the number of simple molecules which combine to give associated molecule, observed molar mass, degree of association(α) can be calculated.
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
