NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 14, “Biomolecules,” is a study material package designed to help students understand the various types of biomolecules, their structures, functions, and their importance in biological systems.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including carbohydrates, lipids, nucleic acids, and proteins. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the classification of biomolecules to their structural features, properties, and functions. It also includes questions on the different types of carbohydrates, lipids, nucleic acids, and proteins, as well as their importance in biological systems.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the various types of biomolecules, their structures, functions, and the importance of enzymes in biochemical reactions.
NCERT Solution / Notes Class 12 Chemistry Chapter 14 Biomolecules with Mind Map PDF Download
Introduction:
In this Unit, the Structures and functions of some of biomolecules will be discuss. The structure and functions of biomolecules inside the living being is studied in biochemistry. Living systems are made up of various complex biomolecules such as carbohydrates, proteins, enzymes, lipids, vitamins, hormones, nucleic acids and compounds for storage and exchange of energy such as ATP, etc.
Carbohydrates:
Classification of Carbohydrates
On the basis of their behaviour upon hydrolysis, carbohydrates can be divided into three main groups :
- Monosaccharides: Monosaccharide is a carbohydrate that cannot be hydrolyzed into a simpler units of polyhydroxy aldehyde or ketone. About 20 monosaccharides are known to occur in nature. e.g., glucose, fructose, ribose etc.
- Oligosaccharides: A carbohydrate which upon hydrolysis yields 2–10 unit of monosaccharide is called oligosaccharide. They are further classified as disaccharides, trisaccharides, etc., depending upon the number of monosaccharides, they provide on hydrolysis. For example, sucrose is a disaccharide which on hydrolysis yields two unit of monosaccharides i.e., glucose and fructose whereas raffinose is a trisaccharide which on hydrolysis yields three unit of monosaccharides i.e., glucose, fructose and galactose.
- Polysaccharides: A high molecular mass carbohydrate which upon hydrolysis yields a large number of monosaccharide units is called polysaccharide e.g., starch, cellulose, glycogen, gums, etc.
(C6H10O5)n + nH2O ⟶ nC6H12O6
Sugar and non-sugars : In general monosaccharides and oligosaccharides, are crystalline solids, soluble in water and sweet to taste, are collectively known as sugars. The polysaccharides, on the other hand, are amorphous insoluble in water and tasteless, are known as non-sugars.
Reducing and non-reducing carbohydrates : The carbohydrates containing free aldehydic or ketonic group can reduce Fehling’s solution and Tollen’s reagent are known as reducing carbohydrates. All monosaccharides whether aldose or ketose are reducing in nature. The carbohydrates in which the reducing parts are not free cannot reduce Fehling’s solution and Tollen’s reagent are known as non-reducing carbohydrates. All polysaccharides like starch, cellulose, glycogen etc. are non-reducing carbohydrates.
- Monosaccharides
If a monosaccharide contains an aldehyde group, it is known as an aldose and if it contains a keto group, it is known as a ketose.
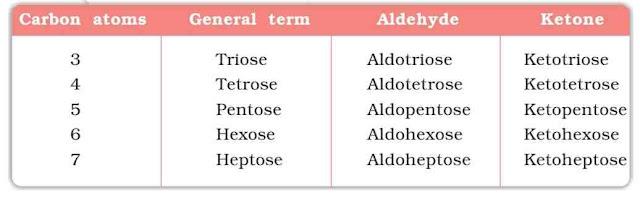
Glucose
Glucose occurs in nature in free as well as in the combined forms. It is present in sweet fruits and honey. Ripe grapes contain ~20% of glucose.
Preparation of Glucose
- From Sucrose (Cane Sugar): When sucrose is boiled with dilute HCl or H2SO4 in alcoholic solution, glucose and fructose are obtained in equimolar proportion.
C12H22O11 + H2O ⟶ C6H12O6 + C6H12O6
- From Starch : When starch is boiled with dilute H2SO4 at 393 K under pressure, glucose is obtained. This is the commercial method for the preparation of glucose.
(C6H10O5)n + nH2O ⟶ nC6H12O6
Structure of Glucose: Glucose is an aldohexose and is the monomer of many larger carbohydrates like starch, cellulose etc. It is the most abundant organic compound on the Earth.
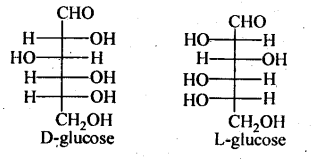
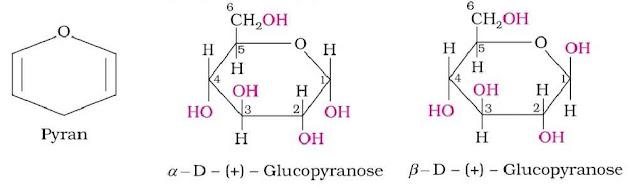
Cyclic Structure of Glucose: It was proposed that glucose can form a six-membered ring in which –OH at C-5 can add to the –CHO group and can form a cyclic hemiacetal structure. This explains the absence of –CHO group and also the existence of glucose in α and β-anomeric forms as
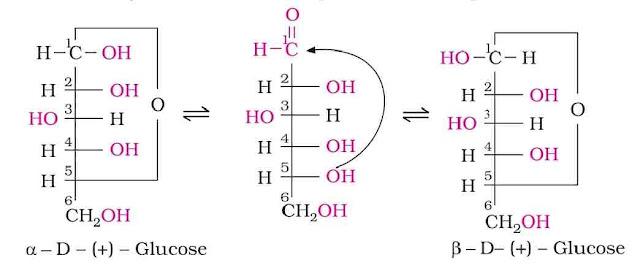
The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C-1, called anomeric carbon and the corresponding α and β-forms are called anomers. It should be noted that α and β-forms of glucose are not mirror images of each other, hence are not enantiomers.
Fructose
Fructose is an important ketohexose. It is obtained by the hydrolysis of sucrose. On the basis of molecular weight determination, elemental analysis and various reaction its molecular formula is found to be C6H12O6 and open chain structure of it can be written as
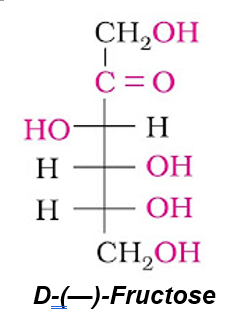
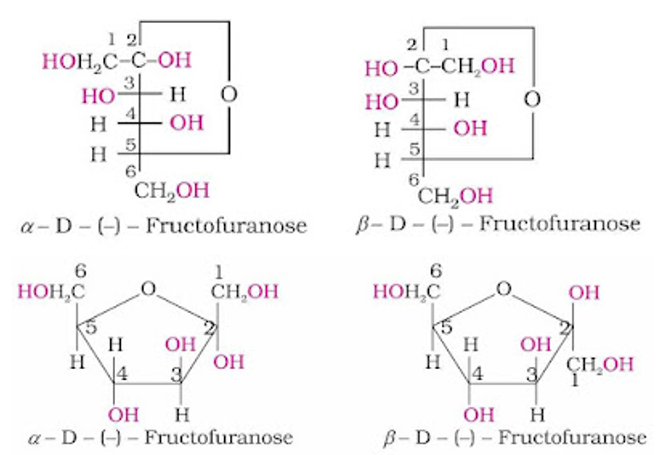
Fructose also exists in two cyclic forms like glucose i.e., α-D-(–) – fructose and β-D- (–) – fructose. The five membered cyclic structure of fructose is formed by the involvement of –OH at C-5 and carbonyl group. The five-membered ring of fructose is named as furanose with analogy to the compound furan.
- Disaccharides
The disaccharides are composed of two units of monosaccharides. On hydrolysis with dilute acids or specific enzymes they give the corresponding monomers.
C12H22O11 + H2O ⟶ C6H12O6 + C6H12O6
In disaccharides the two monosaccharides units are joined together by an oxide linkage formed by the loss of a water molecule and the linkage is known as glycosidic linkage.
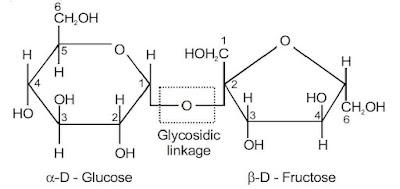
Sucrose
Sucrose is formed by the glycosidic linkage between C-1 of α-D-(+)-glucose and C-2 of β-D-(–) fructose:
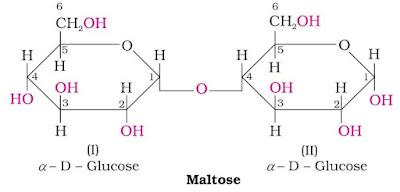
Maltose
Maltose is formed by the glycosidic linkage between C-1 of one glucose unit to the C-4 of another glucose unit.
Lactose
Lactose is found in milk so it is also known as milk sugar. It is formed by the glycosidic linkage between C-1 of α-D-galactose unit and C-4 of β-D-glucose unit. Lactose is a reducing sugar.
Polysaccharides
Polysaccharides are long chain polymer of monosaccharides joined together by glycosidic linkages. For example, starch, cellulose, glycogen etc. They mainly act as the food storage or structural materials.
Starch (C6H10O5)n
Starch is the main storage polysaccharide of plants. High content of starch is found in cereals, roots, tubers and some vegetables. Starch is a polymer of α-D-(+) Glucose coming of two components namely Amylose and Amylopectin.
Amylose is water soluble component, which constitutes about 15 – 20% of starch. It is a straight chain polysaccharide containing α-D-(+)-glucose units joined together by β-glycosidic linkage involving C-1 of one glucose unit and C-4 of the next.
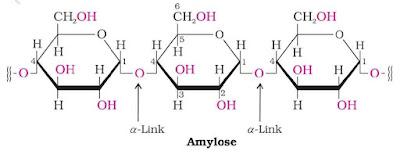
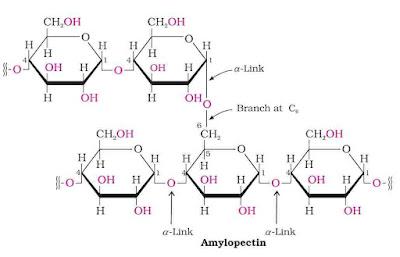
Amylopectin is a branched chain polysaccharide insoluble in water. It constitutes about 80 – 85% starch. It is a branched chain polymer of α-D-glucose units in which chain is formed by C-1 – C-4 glycosidic linkage whereas branching occurs by C-1 – C-6 glycosidic linkage.
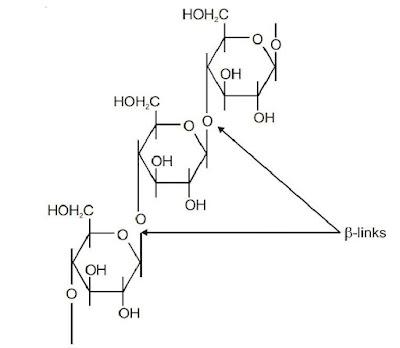
Cellulose
Cellulose is a straight chain polysaccharide composed of only β-D-glucose units. In cellulose there is β-glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose unit. Cellulose occurs mainly in plants and it is the most abundant organic substance in plant kingdom.
Glycogen
Its structure is similar to amylopectin with more branching than in amylopectin. It is also known as animal starch. In body, carbohydrates are stored as glycogen and when the body needs glucose, enzymes break the glycogen down to glucose. Glycogen is present in liver, muscle and brain.
Note : Carbohydrates are essential for life in both plants and animals. Carbohydrates are stored in plant as starch and in animals as glycogen.
Proteins:
Proteins are high molecular mass complex biopolymer of α-amino acids present in all living cells. They occur in every part of the body and form the fundamental basis of structure and functions of life. The term protein is derived from the Greek word “proteios” which means of prime importance. Proteins are the most abundant biomolecules of the living system. Chief sources of proteins are milk, cheese, pulses, peanuts, fish etc.
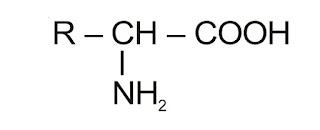
Amino Acids:
The compound containing –NH2 and –COOH functional groups are known as amino acid, depending upon the relative position of –NH2 group with respect to –COOH group, amino acids are classified into α, β, γ, δ and so on amino acid. Hydrolysis of proteins gives only α-amino acids represented as
Essential and non-essential amino acids:
The amino acids which cannot be synthesized in the body are known as essential amino acids which must be taken through diet. The amino acids, which can be synthesized in the body are known as non-essential amino acids.
Peptides
When amino acids are joined together by amide bonds, they form larger molecules called peptides and proteins.
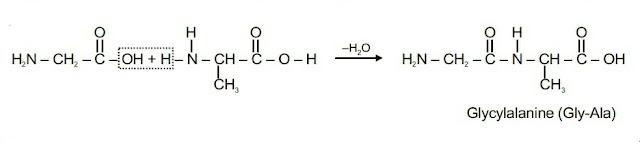
Polypeptide : A dipeptide contains two amino acids linked by one peptide linkage, a tripeptide contains three amino acids linked by two peptide linkages and so on. When number of such amino acids is more than ten, then the products are called polypeptides.
Classification of Protein
On the basis of molecular shape, proteins are classified into two types :
- Fibrous Proteins : When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, then fibre-like structure is formed, known as fibrous proteins. Such proteins are insoluble in water. For example: Keratin, Myosin etc.
- Globular Proteins : When the polypeptide chains coil around to give a spherical shape, the formation of globular protein takes place. Such proteins are usually soluble in water. For example : Insulin, Albumins etc.
Primary, Secondary, Tertiary & Quaternary Structures of Proteins :
- Primary Structure : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein.
- Secondary Structure : The secondary structure of protein refers to the shape in which a long polypeptide chain can exist. They are found to exist in two different types of structure namely α-helix and β-pleated sheet structure.
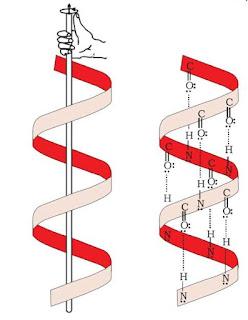
- Tertiary Structure : The tertiary structure of proteins represents overall folding of the polypeptide chains i.e., further folding of secondary structure. It gives rise to two major molecular shapes namely fibrous and globular.
- Quaternary Structure : Some of the proteins are composed of two or more polypeptide chains referred to as sub units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.
Denaturation of Proteins:
The loss in biological activity of a protein due to unfolding of globules and uncoiling of helix is called denaturation of protein. During denaturation secondary and tertiary structures are destroyed but primary structure remains intact. The coagulation of egg white on boiling is a common example of denaturation.
Enzymes:
Colloidal solution of protein which works as biological catalyst is known as enzyme. All enzymes are globular proteins. Zymase, Invertase, Maltase, Lactase, Emulsin, Urease, Pepsin, Trypsin, α-Amylase etc are the example of enzyme.
Note : The enzymes work best at an optimum temperature range of 298 K to 313 K. Their activity decreases with decrease or increase in temperature and stops at 273 K.
Properties of enzymes
- Enzymes are required only in small amounts.
- They are highly specific.
- Enzymes are efficient catalyst: they speed up reaction.
- They work at optimum pH, at optimum temperature.
- Their mechanism is controlled by various mechanisms and stopped by various organic and inorganic compounds.
- The action of enzymes follows lock and key mechanism .however enzyme action is inhibited by certain organic molecules called inhibitors.
It is of two types:
- Cofactor: The prosthetic group which is covalently attached with the enzyme molecule is known as cofactor.
- Coenzyme: The prosthetic group which get attached to the enzyme at the time of a reaction is known as coenzyme.
Nomenclature of Enzymes
Enzymes are usually named by adding the suffix ‘ase’ to the root name of the substrate, e.g., urease, maltase, invertase, etc.
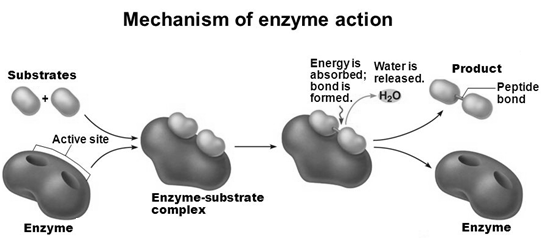
Mechanism of Enzyme Action
- There is a lock and key arrangement between the an enzyme and a substrate.
- Substrates bind at active site, temporarily forming an enzyme-substrate (E-S) complex.
- The E-S complex undergoes internal rearrangements that form the product.
- The enzyme gets regenerated for the next molecule of the substrate.
Vitamins:
Vitamins are organic compounds which are essential for normal growth of life for animals, some bacteria and micro organism. Vitamins are not synthesized by animals (except vitamin D). Vitamins are supplied to the organism through food. They are essential dietary factor.
• Vitamins are a group of organic compounds which are essential for normal growth and nutrition and are required in very small amounts for maintaining optimum growth and a good health.
• Their absence causes specific deficiency diseases.
• Most of the vitamins cannot be synthesised in our body but plants can synthesise almost all of them.
• Vitamin D is an exception because it can be made in the skin from exposure to sunlight.
Classification of Vitamins
On the basis of solubility in water, vitamins are classified into the following two types:
• Fat soluble vitamins: Vitamins A, D, E and K are oil soluble.
• Water soluble vitamins: The group includes Vitamins B and C. These are stored in much lesser amounts in the cells.
Note: Vitamin H (Biotin) is an exception, since it is neither soluble in water nor in fat.
Some important Vitamins, their Sources and their Deficiency Diseases are dictated in the table given below
| Name of Vitamin | Important Sources | Deficiency Diseases |
| Vitamin A | Fish liver oil, Milk, butter, egg yolk, green and yellow vegetables. | Night blindness, Xerophthalmia (hardening of cornea of eye). |
| Vitamin B1 | Yeast, milk, green vegetables, cereals, fruits, egg yolk. | Beriberi (loss of appetite, retarded growth) |
| Vitamin B2 | Egg yolk, liver, milk, green leafy vegetables. | Cracked lips, sore tongue, digestive disorders and burning sensation of the skin. |
| Vitamin B6 | Milk, egg yolk, cereals, yeast, legumes. | Nervous disturbances and convulsions. |
| Vitamin B12 | Meat, fish, kidney, eggs. | Pernicious anaemia (RBC deficient in haemoglobin) |
| Vitamin C | Citrus fruits, amla and green leafy vegetables. | Scurvy (bleeding gums) |
| Vitamin D | Exposure to sunlight, fish and egg yolk | Rickets (bone deformities in children) and osteomalacia (soft bones and joint pain in adults) |
| Vitamin E | Milk, ghee, vegetable oils like wheat germ oil, sunflower oil, cotton seed oil. | Increased fragility of RBCs and muscular weakness |
| Vitamin H | Milk, yeast, liver, kidney. | Loss of hair, dermatitis. |
| Vitamin K | Green leafy vegetables, fish, meat, cereals. | Increased blood clotting time |
Nucleic Acid:
The particles in nucleus of the cell, responsible for heredity are called chromosomes which are made up of proteins and another type of biomolecules called nucleic acid. These are natural biopolymers made of nucleotide units i.e., polynucleotides. Nucleic acid contain the elements carbon, oxygen, nitrogen and phosphorous.
Nucleic acids
- Nucleic acids are the polymers in which nucleotides are monomers. These are biomolecules present in nuclei of all living cells in the form of nucleoproteins .They are also called as polynucleotides .
They help in the role of transmission of hereditary characters and synthesis of proteins.
Each nucleotide consists of 3 parts:
- A pentose sugar
- A nitrogenous base
- A phosphate group
- The nitrogenous base and a pentose sugar are called as nucleoside.
Nitrogenous bases are of two types: Purines and Pyrimidines
- Purines: adenine and guanine
- Pyrimidines: cytosine , thiamine and uracil
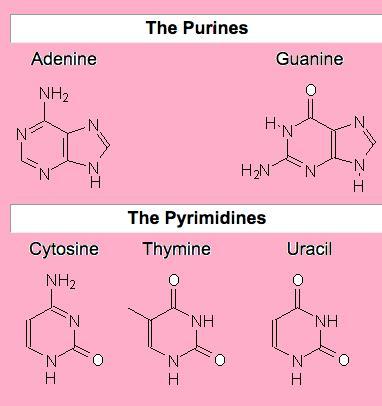
Please note that Purines and Pyrimidines are linked together by hydrogen bonds
- Adenine always bond with thiamine by double bond or vice versa.
- Cytosine always pairs with guanine by triple bond or vice versa.
Types of nucleic acids
- Deoxyribonucleic acid (DNA)
- Ribonucleic acid (RNA)
DNA
- It occurs in nucleus of cell. It has double stranded helical structure
DNA contains:
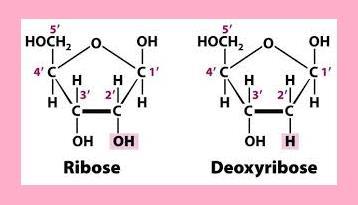
- Deoxyribose sugar
- Nitrogenous bases :
- Purines (adenine and guanine ), Pyrimidines (thiamine and cytosine )
- A phosphate group
- It can undergo replication
- It helps in transfer of genetic information from parents to offspring
RNA
- It occurs in cytoplasm of cell
It consist of:
- Ribose sugar
- Nitrogenous base
- Purines: adenine and guanine
- Pyrimidines: cytosine and uracil
- A phosphate group
- It has a single strand helical structure
- It doesn’t undergo replication
- It controls synthesis of proteins
The structure of deoxyribose and ribose sugar is given:
Structure of nucleic acids
- Primary structure
- The nucleic acids are formed by the condensation of thousands of molecules of nucleotides.
- On hydrolysis the nucleotides produces phosphoric acid and nucleoside .it means nucleosides on hydrolyses form Purine and Pyrimidines base and sugar moiety.
- A nucleic acid– NH3ànucleotides –aq NH3à nucleosides + phosphoric acid –dilute HCL–àPurines + Pyrimidines + sugar.
- Nucleotides are building blocks of nucleic acids.
- These nucleotides are linked together with one another in a particular sequence, phosphate groups forming bridges between C-5 of the sugar residue of the one nucleoside and C-3 of the sugar residue of the other nucleoside.
- The manner in which the sugar, phosphate and bases are linked with one another in nucleic acids is known as primary structure of nucleic acids.
- Secondary structure:
- Watson and Crick explained the double helix structure of DNA. The nucleotides in each strand are connected by phosphate ester bond and bases of one strand by hydrogen bonds.
- Adenine pairs with thiamine through two double hydrogen bonds whereas cytosine pairs with guanine by triple hydrogen bonds.
- The two strands of DNA are complementary to each other that is if one side there is Purine then on other side at same position Pyrimidine is present. For example if base sequence on strand is ACTCGCCA, then on the other strand the sequence will be complementary that is: TGAGCGGT
- The primary and secondary structure is shown below:
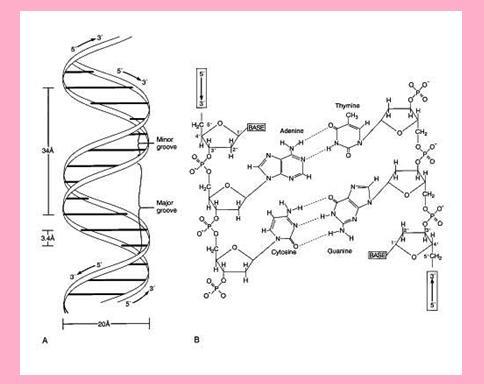
Watson and Crick model of DNA
Functions of nucleic acids
- Replication: The genetic information of cell is contained in the sequence of bases A, T, C and G in DNA molecule .In the division of cell, DNA molecules replicate and makes exact copies of themselves so that each daughter cell will have DNA identical to that of the parent cell.
- Protein synthesis: The specific information coded on DNA has to be translated and expressed in the form of synthesis of specific proteins which performs various functions in the cell. This synthesis is done in two steps:
- Transcription and translation.
- Gene and genetic code: Each segment of DNA molecule that codes for specific protein or a polypeptide is known as The relationship between nucleotides triplets and the amino acids are called the genetic code .This is gene and genetic code.
- Mutation: It is a chemical change in DNA molecule, which leads to the synthesis of proteins with a changed amino acid sequence.
- These changes are caused by radiation, viruses or chemical agents.
- The majority of changes in DNA are replicated by special enzymes in the cell, but if there is failure to repair by the enzymes then it can cause mutation.
Hormones:
Hormones are molecules that act as intercellular messengers. These are produced by endocrine glands in the body and are poured directly in the blood stream which transports them to the site of action. Hormones have several functions in the body. They help to maintain the balance of biological activities in the body. Testosterone is the major sex hormone produced in males.
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
