NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 7, “The p-Block Elements,” is a study material package designed to help students understand the properties and uses of the elements belonging to the p-block of the periodic table.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including the electronic configuration, trends in physical and chemical properties, and the preparation and properties of some important compounds of the p-block elements. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the general trends in the properties of the p-block elements to the preparation and properties of some important compounds such as boron, silicon, and nitrogen. It also includes questions on the uses and applications of the p-block elements in various industries.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the electronic configuration and trends in the physical and chemical properties of the p-block elements, the preparation and properties of some important compounds, and their uses and applications.
NCERT Solution / Notes Class 12 Chemistry Chapter 7 The p-Block Elements with Mind Map PDF Download
Sulphur and its Compounds | Allotropic Forms of Sulphur
Sulphur forms a variety of allotropes. The most common allotropes are yellow rhombic and monoclinic sulphur. Rhombic sulphur is more stable at room temperature. It gets transformed to monoclinic sulphur when heated above 369 K.
Rhombic Sulphur
- This allotrope is yellow in colour. Its melting point is about 385.8 K and specific gravity is 2.06.
- Rhombic sulphur crystals are formed when the solution of roll sulphur in CS2 is evaporated.
- It is insoluble in water but dissolves to some extent in benzene, alcohol and ether. It is more soluble in CS2.
Monoclinic Sulphur
- Its melting point is 393 K and its specific gravity is 1.98. It is soluble in CS2.
- This form of sulphur is prepared by melting rhombic sulphur in a dish and cooling, till a crust is formed.
- Two holes are made in the crust and the remaining liquid is poured out. After removing the crust, colourless needle-shaped crystals of sulphur are formed.
- It is stable above 369 K and transforms into sulphur below 369 K.
- Also, we can say that the sulphur is stable below 369 K and transforms into sulphur above this. At 369 K, both forms are stable. This temperature is called transition temperature.
- Rhombic and monoclinic sulphur have S8 molecules. These S8 molecules are packed to give different crystal structures. The S8 ring in both forms is puckered and has a crown shape.
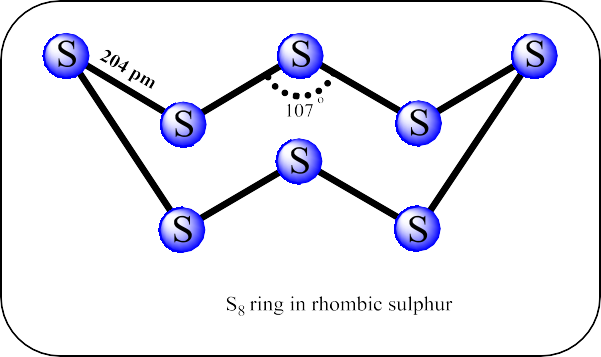
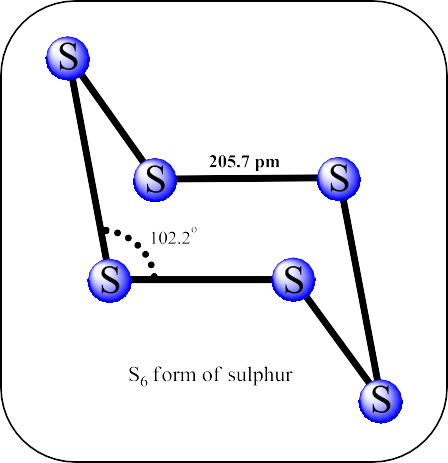
- In the cyclo-S6 form, the molecule is in the chair shape.
- At very high temperatures (~1000 K); S2 is paramagnetic like O2.
Sulphur Dioxide Preparation
- It can be prepared in the laboratory with the action of metallic sulphate on a dilute acid.
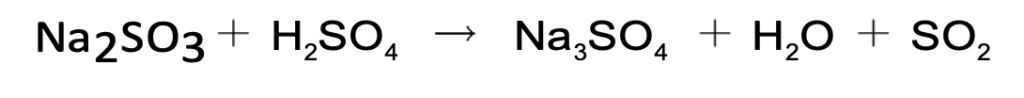
- Sulphur dioxide is formed together with a trace amount of sulphur trioxide (6–8%) when sulphur is burnt in air or oxygen:

- In the laboratory, sulphite is treated with dilute sulphuric acid to give sulphur dioxide.
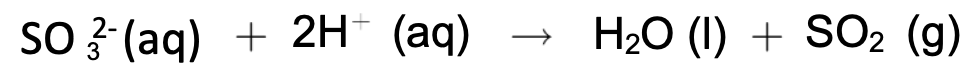
- It is also produced as a by-product of the roasting of sulphide ores.
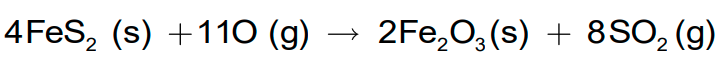
- The gas is first dried and is liquefied under pressure and stored in steel cylinders.
Properties
Physical Properties
- Sulphur dioxide is one of the gases which can be easily liquefied.
- Sulphur dioxide is a colourless gas with pungent smell and is highly soluble in water.
- It liquefies at room temperature under a pressure of 2 atmospheres and boils at 263 K.
- When sulphur dioxide is passed through water, it forms a solution of sulphurous acid.
Chemical Properties
- It reacts with sodium hydroxide solution to give sodium sulphite, which then reacts with excess of sulphur dioxide to form sodium hydrogen sulphite.
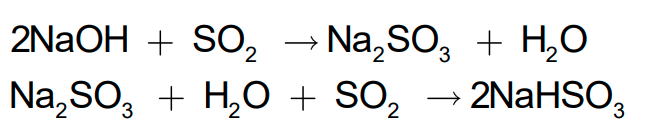
- When sulphur dioxide reacts with water or alkali, its behaviour is similar to that of carbon dioxide.
- Sulphur dioxide reacts with chlorine in the presence of charcoal (which acts as a catalyst) to give sulphuryl chloride SO2Cl2.
- It is oxidised to sulphur trioxide by oxygen in the presence of vanadium (V) oxide catalyst.
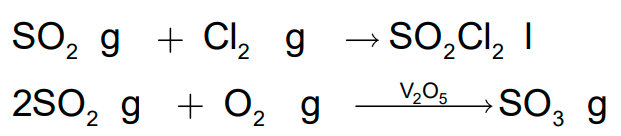
- Under moist conditions, sulphur dioxide behaves as a reducing agent. For example, it converts iron (III) ions to iron (II) ions and decolourises acidified potassium permanganate (VII) solution.
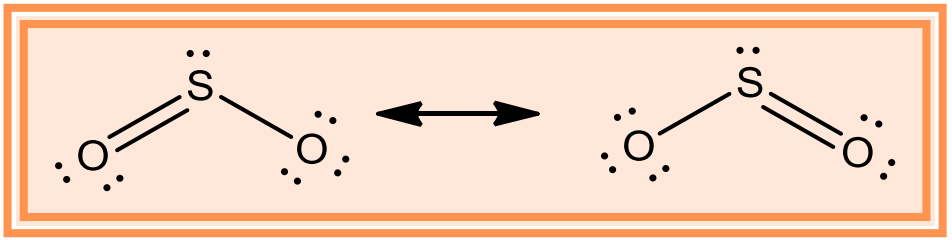
- The molecule of SO2 is angular. It is a resonance hybrid of the two canonical forms:
Uses
- As a bleaching agent
- In refining petroleum and sugar
- In bleaching wool and silk
- As an anti-chlor, disinfectant and preservative
- In the manufacture of sulphuric acid, sodium hydrogen sulphite and calcium hydrogen sulphite (industrial chemicals)
- Liquid SO2 is used as a solvent to dissolve several organic and inorganic chemical
Oxoacids of Sulphur
- Sulphur dioxide is a strong oxidising agent.
- Sulphur forms several oxoacids such as H2SO3, H2S2O3, H2S2O4, H2S2O5, H2SxO6 (x = 2−5), H2SO4, H2S2O7, H2SO5 and H2S2O8.
- Some of these acids are unstable and cannot be isolated.
- They commonly occur in the form of an aqueous solution or in the form of their salts.
Sulphuric Acid Preparation
- Sulphuric acid is one of the most important industrial chemicals.
- Sulphuric acid is manufactured by the contact process which involves three steps:
Burning of sulphur or sulphide ores in air to generate SO2
Conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5)
Absorption of SO3 in H2SO4 to give oleum (H2S2O7)
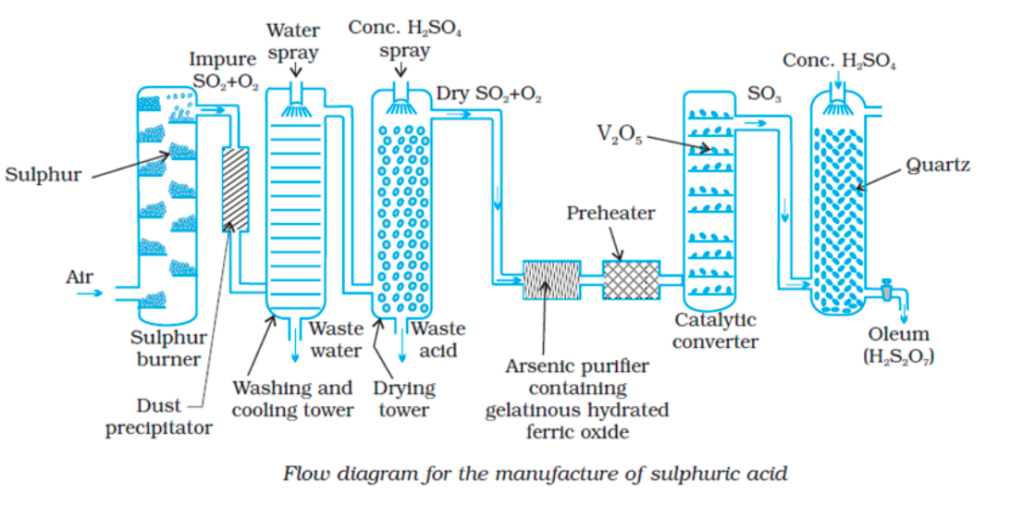
- SO2 produced by this process is purified by removing dust and other impurities such as arsenic compounds.
- The major step in the manufacture of H2SO4 is the catalytic oxidation of SO2 with O2 to give SO3 in the presence of V2O5 (catalyst).
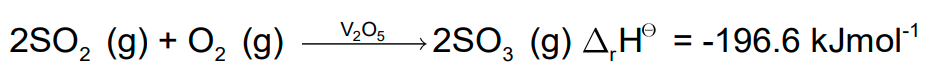
- The reaction is exothermic and reversible. The forward reaction leads to a decrease in volume.
- Low temperature and high pressure are favourable conditions for maximum yield.
- But the temperature should not be very low; otherwise the rate of reaction will become slow.
- In actual practice, the plant is operated at a pressure of 2 bar and 720 K.
- SO3 gas from the catalytic converter is absorbed in concentrated H2SO4 to produce oleum. Dilution of oleum with water gives H2SO4 of the required concentration.
- In the industry, two steps are carried out simultaneously to make the process a continuous one and to reduce the cost.
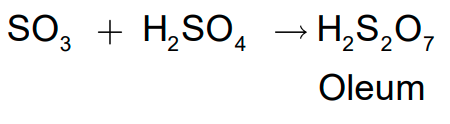
- Sulphuric acid obtained by the contact process is 96–98% pure.
Properties
- Sulphuric acid is a colourless, dense, oily liquid with a specific gravity of 1.84 at 298 K.
- The acid freezes at 283 K and boils at 611 K.
- It is highly exothermic in the presence of water. It dissolves in water with the evolution of a large quantity of heat.
- Hence, care must be taken while preparing sulphuric acid solution from concentrated sulphuric acid.
- The concentrated acid must be added slowly into water with constant stirring.
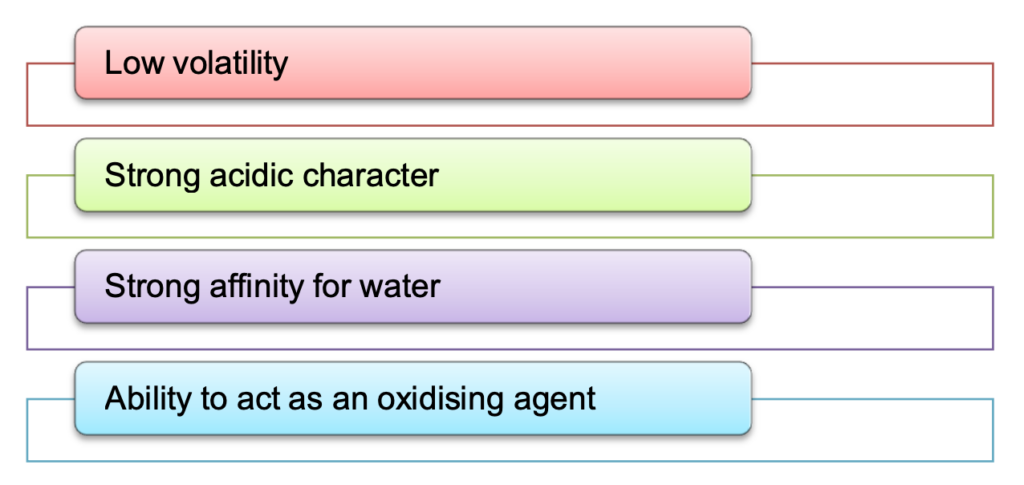
- Chemical reactions of sulphuric acid are as a result of the following characteristics:
- In an aqueous solution, sulphuric acid ionises in two steps.
- The larger value of Ka1 (Ka1 > 10) means that H2SO4 is largely dissociated into H+ and HSO4.
- Greater the value of the dissociation constant (Ka), the stronger is the acid.
- The acid forms two series of salts—normal sulphates (sodium sulphate and copper sulphate) and acid sulphates (sodium hydrogen sulphate).
- Sulphuric acid can be used to manufacture more volatile acids from their corresponding salts because of its low volatility.
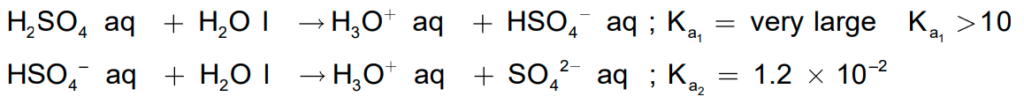
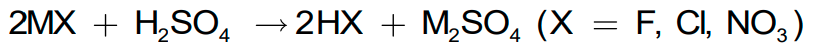
- Concentrated sulphuric acid is a strong dehydrating agent.
- Many wet gases can be dried by passing them through sulphuric acid, provided the gases do not react with the acid.
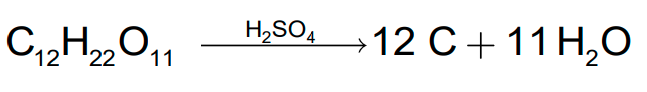
- Sulphuric acid removes water from organic compounds; it is evident by its charring action on carbohydrates.
- Hot concentrated sulphuric acid is a moderately strong oxidising agent.
- In this respect, it is intermediate between phosphoric acid and nitric acid.
- Both metals and non-metals are oxidised by concentrated sulphuric acid, which is reduced to SO2.
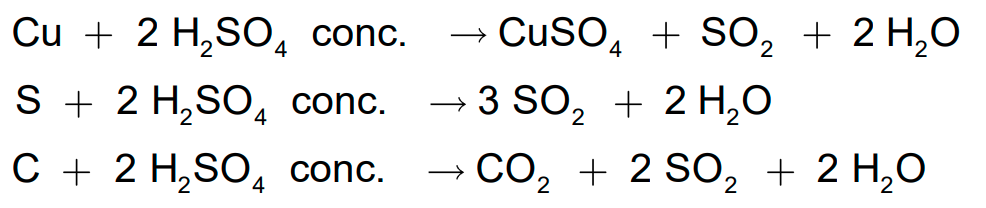
Uses
- Sulphuric acid is a very important industrial chemical as many other chemicals can be prepared from it.
- Primary use of sulphuric acid is in the synthesis of fertilisers.
- The industrial strength can be judged by the quantity of sulphuric acid it produces and consumes.
- It is needed for the manufacture of hundreds of other compounds and in many industrial processes.
- Bulk of sulphuric acid produced is used in the manufacture of fertilisers (ammonium sulphate and superphosphate).
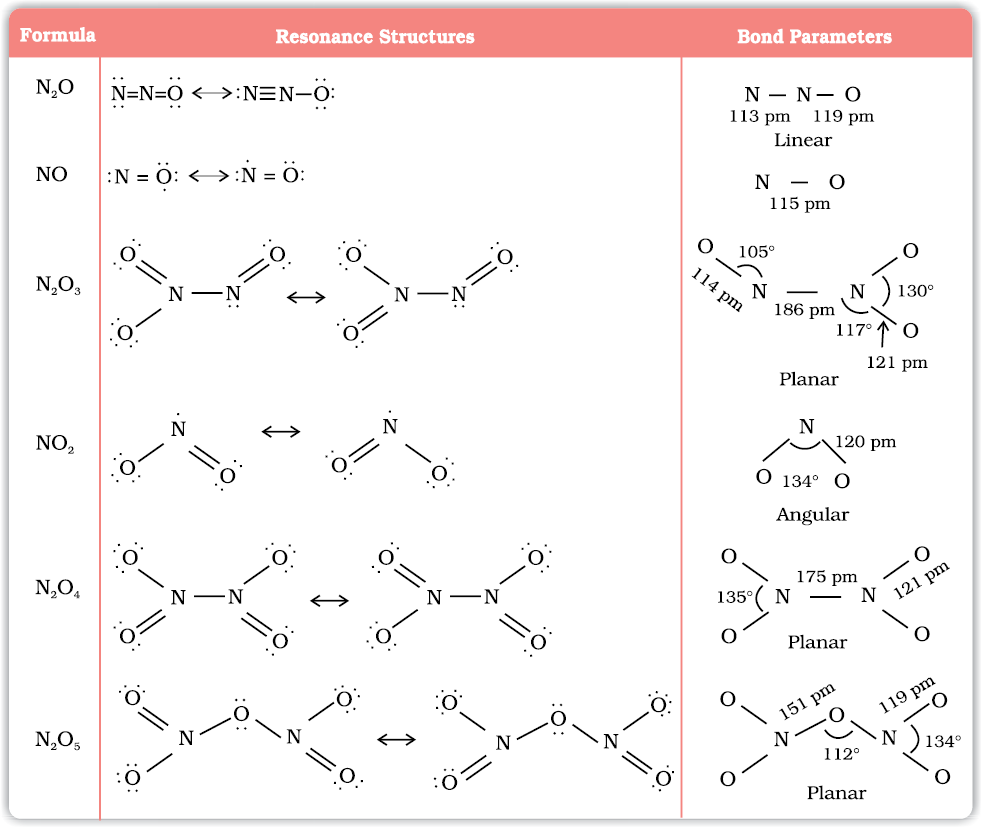
Oxides of Nitrogen
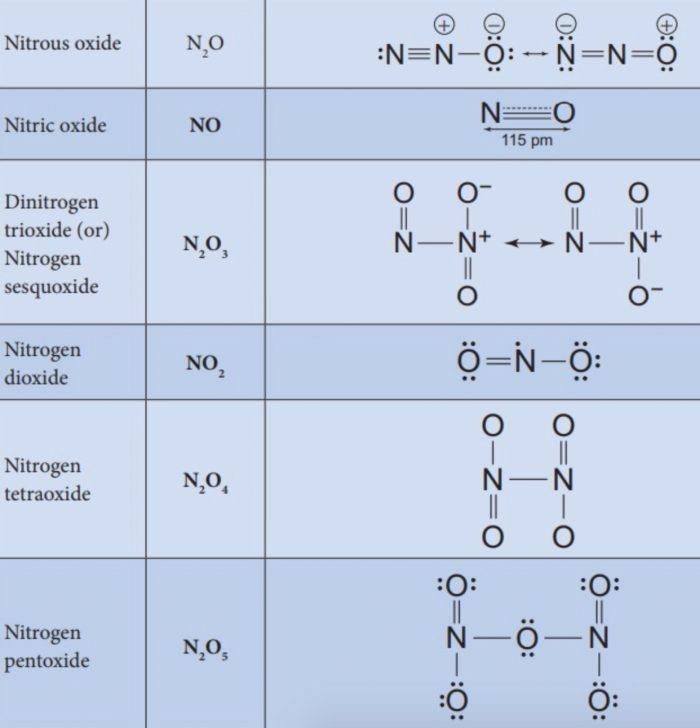
Nitrogen combines with oxygen under different conditions to form a number of binary oxides which differ with respect to the oxidation state of the nitrogen atom. They range from N2O (oxidation state of N +1) through NO (+2), N2O3 (+3), N2O4 (+4) to N2O5(5). The tendency to form pπ – pπ multiple bonds dictates the structures of oxides.
(1) Nitrous Oxide (N2O)
(a) It is prepared by heating ammonium nitrate.
NH4NO3 —–> N2O + 2H20
(b) It is a colourless unreactive gas having faint pleasant smell. It is also known as laughing gas because it causes hysterical laughter when inhaled in minor quantities.
(c) It is a neutral oxide and reacts with sodamide to form sodium azide.
N2O + 2NaNH2 ——–> NaN3 + NH3 + NaOH
Sodamide Sodium azide
(d) In small amounts, it acts as an anaesthetic for minor operations.
(e) It decomposes into nitrogen and oxygen at 873 K.
2N2O ——> 2N2 + O2
Therefore, it supports the combustion acting as a source of oxygen.
(2) Nitric Oxide (NO)
(a) It is prepared by the catalytic oxidation of ammonia at 1100 K in the presence of platinum.
4NH3 + 5O2 ——->4NO + 6H2O
(b) It can also prepared by the reaction of nitric acid on copper as :
3Cu + 8HNO3 ——> 3Cu(NO3)2 + 2NO + 4H2O
(c) It can also be prepared by the reduction of sodium nitrite with ferrous sulphate in the presence of sulphuric acid.
2NaNO2 + 2FeSO4 + 3H2SO4 ——-> Fe2(SO4)3 + 2NaHSO4 + 2H2O + 2NO
(d) It is a neutral oxide
(e) It is a colourless gas. It has odd number of electrons (11 valence electrons) and therefore, it is paramagnetic in the gaseous state. However, in the liquid and solid states, it forms a loose dimer in such a way that the magnetic effects of two unpaired electrons are cancelled out. The molecule is diamagnetic.
(f) Nitric oxide readily reacts with oxygen to give brown fumes of nitrogen dioxide.
2NO (g) + O2(g) ——–> 2NO2 (g)
(g) Nitric oxide readily forms complexes with transition metals.
For example: Fe2+ combines with NO to form the complex [Fe(H2O)5NO]2+ which is responsible for brown ring test for nitrates.
(h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K)
2NO (g) —–> N2 (g) + O2 (g)
Dinitrogen Trioxide (N2O3)
(1) It is prepared by cooling equimolar quantities of nitric oxide and nitrogen dioxide to below 253 K.
NO(g) + NO2(g) ⇔ N2O3
(2) It can also be prepared by reacting nitric oxide and dinitrogen tetraoxide at 250 K.
2NO + N2O4 ——> 2N2O3
Properties of Dinitrogen Trioxide
(1) It is a blue solid and is acidic in nature. It is anhydride of nitrous acid (HNO2).
N2O3 + H2O —-> 2HNO2
(2) It exists in the pure form only in the solid state at very low temperatures.Above its melting point (273 K) it dissociates to NO and NO2.
N2O3 ——-> NO + NO2
Nitrogen Dioxide (NO2)
It is prepared by heating dried lead nitrate in a steel reaction vessel.
2Pb (NO3)2 ——-> 2PbO+ 4NO2 + O2
It is also an odd electron molecule and in the gas phase, it exists in equilibrium with N2O4 as :
N2O4 ⇔ 2 NO2
Above 415 K it contains mainly NO2 and at 250 K, it consists of mainly N2O4
2 NO2 ⇔ N2O4
Dinitrogen Pentoxide (N2O5)
(1) It is prepared by dehydrating the concentrated nitric acid with phosphorus pentoxide.
4HNO3 + P4O10 —–> 2N2O5 + HPO3
(2) N2O5 exists as colourless solid below 273K. As the temperature rises, the colour changes to yellow due to the partial decomposition of colourless N2O5 to brown NO2.
2 N2O5 ——–> 4NO2 + O2
(3) At 303K, the crystals melt giving a yellow liquid which decomposes at 313K to give NO2.
(4) N2O5 acts as a strong oxidising agent and oxidises iodine to I2O5
NO and NO2 are used in the manufacture of nitric acid and nitrate fertilizers Liquid N2O4 is also used as an oxidiser for the rocket fuels in missiles and space vehicles.
(5) NO causes a pollution problem in atmosphere due to its poisonous nature. Its vapours are emitted in the atmosphere during the burning of oil and coal.
Nitric Acid (HNO3)
The common oxoacids of nitrogen are given below :
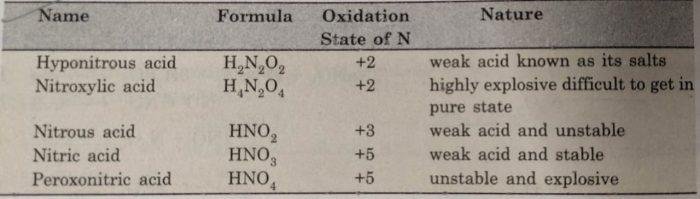
Nitric acid is a very strong oxidising agent.Nitrogen shown an oxidation state of +5 in nitric acid.
Laboratory Preparation of Nitric Acid
In the laboratory, nitric acid can be prepared by heating sodium or potassium nitrate with concentrated sulphuric acid to about 423-475 K.
NaNO3 + H2SO4 ——> NaHSO4 + HNO3
Anhydrous nitric acid can be obtained by distillation of concentrated aqueous nitric acid with P4010.
Manufacture of Nitric Acid
Nitric acid is commonly manufactured by Ostwald process in which it is prepared by the catalytic oxidation of ammonia by atmospheric oxygen. The reaction is carried out at about 500 K and 9 x 105 Pa (9 bar) pressure in the presence of Pt or Rh gauge as catalyst.
4NH3(g) + 502(g) ——> 4NO(g) + 6H20(g) ΔH =- 90.2 kJ
Pt/Rh gauge, 500K, 9 bar
Nitric oxide thus formed combines with oxygen to form nitrogen dioxide.
2NO(g) + O2 (g) ——> 2 NO2 (g)
Nitrogen dioxide so formed, dissolves in water to give nitric acid.
3NO2 (g) + H2O(l) —–> 2HNO3(aq) + NO(g)
Dilute nitric acid is further concentrated by dehydration with concentrated sulphuric acid to get about 98% acid.
Properties of Nitric Acid
Physical Properties
1) Pure nitric acid is a colourless liquid.
2) It has boiling point 355.6 K and freezing point 231.4 K.
3) laboratory grade nitric acid contains about 68% of HNO3 by mass and has a specific gravity of 1.504.
4) The impure acid is generally yellow due to the presence of nitrogen dioxide as impurity. Nitric acid containing dissolved nitrogen dioxide is known as fuming nitric acid.
5) It has a corrosive action on skin and produces painful blisters.
Chemical Properties
(1) Acidic character:
It is one of the strongest acids because it is highly ionised in aqueous solution giving hydronium and nitrate ions.
2HNO3(aq) +H2O (l) ——> H3O+ + NO3¯(aq)
It turns blue litmus red. It forms salts with alkalies, carbonates and bicarbonates.
NaOH + HNO3 —-> NaNO3 + H2O
Na2CO3 + HNO3 —-> 2NaNO3 + H2O + CO2
NaHCO3 + HNO3 —-> NaNO3 + H2O + CO2
(2) Action on metals:
With the exception of gold and platinum, nitric acid attacks all metals forming a variety of products. The product depends upon the nature of metal, the concentration of acid and temperature.
(A) Metals that are more electropositive than hydrogen (Mg, Al, Mn, Zn, Fe, Pb, etc.). In this case nascent hydrogen is liberated which further reduces nitric acid.
M + 2HNO3 ——> M(NO3)2 + 2H
HNO3 + H —-> Reduction product + H2O
The principal product is NO2, with conc. HNO3, N2O with dil. HNO3, and ammonium nitrate with very dil. HNO3.
For example: Zn reacts as:
(a) Using concentrated nitric acid (forms nitrogen dioxide)
Zn + 2HNO3 —–> Zn (NO3)2 + 2H
HNO3 + H —–> NO2 + H2O] x 2
—————————————————-
Zn + 4HNO3 —-> Zn (NO3)2 + 2NO2 + 2H2O
(b) Using dilute nitric acid (forms nitrous oxide)
Zn + 2HNO3 —–> Zn(NO3) + 2H ] × 4
2 HNO3 + 8 H —–> NO2 + 5 H2O
—————————————————-
Zn + 10HNO3 —-> 4Zn (NO3)2 + NO2 + 5H2O
(c) Using very dilute nitric acid (forms ammonium nitrate)
Zn + 2HNO3 —–> Zn (NO3)2 + 2H] × 4
HNO3 + 8H —–> NH3 + 3 H2O
NH3 + HNO3 ——–> NH4NO3
———————————————————-
4Zn + 10 HNO3 —–> 4Zn (NO3)2 + NH4NO3 + 3 H2O
(B) Metals which are less electropositive than hydrogen (Cu, Bi, Hg, Ag). In this case nascent hydrogen is not liberated.
HNO3 → Reduction product + H2O + [0]
Metal + (O) + HNO3→ Metal nitrate + H2O
The principal product is NO2 with conc. HNO3 and NO with dil. HNO3
For example: Cu reacts as
(a) Using concentrated nitric acid
2HNO3 ——> 2NO2 + H2O + [O]
Cu + O + 2HNO3 ——–> Cu (NO3)2 + H2O
—————————————————-
Cu + 4HNO3 → Cu (NO3)2 + 2NO2 + 2H2O
(b) Using dilute nitric acid
2HNO3 —–> 2NO + H2O + 3[O]
Cu + O + 2HNO3 –> Cu (NO3)2 + H2O] x 3
——————————————————-
3Cu + 8HNO3 ——-> Cu (NO3)2 + 2NO + 4H2O
Hg + 4HNO3 —–> Hg (NO3)2 + 2NO2 + 2H2O
6Hg + 8HNO3 —–> 3Hg2(NO3)2 + 2NO + 4H2O
Ag + 4HNO3 —–> AgNO3 + NO2 + 2H2O
3 Ag + 4 HNO3 —–> 3 AgNO3 + NO + 2H2O
(c) Action on noble metals
Noble metals like gold and platinum are not attacked by nitric acid. However, these metals are attacked by aqua regia (3 parts conc. HCl and 1 part conc. HNO3) forming their chlorides.
NaOH + HNO3 —–> NaNO3 + H2O
HNO3 + 3 HCl ——> NOCl + 2 H2O + 2 Cl
Nitrosyl chloride
Au + 3Cl —> AuCl3
Pt + 4Cl —-> PtCl4
(3) Oxidising nature -Oxidation of non-metals and compounds.
Nitric acid behaves as a strong oxidising agent. It has a tendency to give nascent oxygen as:
2HNO3 —–> 2NO2 + H2O + O
(conc.)
2HNO3 —–> 2NO + H2O + 3 [O]
Therefore, nitric acid oxidises many non-metals and compounds.
(A) Oxidation of non-metals:
Dilute nitric acid has no action on non-metals like carbon, sulphur, phosphorus, etc. However, concentrated nitric acid oxidises many non-metals.
For example
(1) Nitric acid oxidises sulphur to sulphuric acid
2HNO3 ——> 2NO2 + H2O+ O] x 3
1/8 S8 + H2O + 3O —–> H2SO4
——————————————————————
1/8 S8 + 6HNO3 ——–>H2SO4 + 6NO2 + 2H2O
———————————————————
S8 + 48HNO3 ——–> 8 H2SO4 + 48NO2 + 12H2O
———————————————————-
(ii) Nitric acid oxidises carbon to carbonic acid
2HNO3 —–> 2NO2 + H2O + O] x 2
C + H2O+ 2O —-> H2CO3
————————————————–
C+4HNO3 —–> H2CO3 + 4NO2 + 2H2O
————————————————–
(iii) Nitric acid oxidises phosphorus to phosphoric acid
2HNO3 —–> 2NO2 + H2O + O] x 5
2P + 3H2O + 5O —-> 2 H3PO4
—————————————–
2P+ 10HNO3 —–> 2 H3PO4 + 10 NO2 + 2 H2O
p + 5 HNO3 —> H3PO4 + 5 NO2 + H2O
P4 + 20 HNO3 —-> H3PO3 + 20 NO2 + 4 H2O
(iv) It oxidises iodine to iodic acid.
2HNO3 ——> 2NO2 + H2O + O] × 5
I2 + H2O + 5O —> HIO3
———————————————————
I2 + 10HNO3 ——–> 2 HIO3 + 10 NO2 + 4 H2O
———————————————————
(v) Nitric acid oxidises arsenic to arsenic acid.
2HNO3 ——> 2NO2 + H2O + O] × 5
2As + 3H2O + 5O ——> 2H3AsO4
———————————————————-
2As + 10HNO3 ——> 10NO2 + 2H3AsO4 + 2H2O
As + 5HNO3 ——> 5NO2 + H3AsO4 + H2O
(B) Oxidation of compounds
Dil ute as well as concentrated nitric acid oxidises a number of compounds.
(1) Nitric acid oxidises hydrogen sulphide to sulphur.
dil HNO3:
3H2S + 2 HNO3 —–> 2 NO + 4 H2O + 3S
Conc HNO3
3H2S + 2 HNO3 —–> 2 NO2 + 4 H2O + S
(2) Nitric acid oxidises sulphur dioxide to sulphuric acid
dil HNO3
3SO2 + 2HNO3 + 2H2O —> 3 H2SO4 + 2 NO
conc. HNO3
SO2 + 2 HNO3 ——-> H2SO4 + 2 NO2
(3) Nitric acid oxidises ferrous sulphate to ferric sulphate
dil HNO3
6FeSO4 + 2HNO3 + 3H2SO4 ——> 3Fe2 (SO4)3 + 2NO + 4 H2O
conc. HNO3
2FeSO4 + 2HNO3 +3H2SO4 ——> 3Fe2 (SO4)3 + 2NO2 + 4 H2O
(4) Action on organic compounds
Nitric acid also reacts with organic compounds.
For example: sucrose (cane sugar) is oxidised to oxalic acid by nitric acid.
C12H22 O11 + 36 HNO3 –> 6 (COOH)2 + 36NO2 + 23 H2O
In the presence of sulphuric acid, nitric acid reacts with aromatic compounds forming nitro compounds. This process is called nitration.
For example: it reacts with benzene to form nitrobenzene.
C6H6 + HNO3 —-> C6H5NO2 + H2O
Similarly, phenol reacts with nitric acid in the presence of H2SO4 to give trinitrophenol (known as picric acid).
Nitric acid attacks proteins giving a yellow nitro compound known as xantho protein. Therefore, nitric acid stains skin and renders wool yellow.
Structure
Gaseous nitric acid has planar structure. Nitrate ion, NO3¯ has also planar symmetrical structure

Brown Ring test for NO3¯ ion
Nitrates give brown ring test with Fe2+ ions in the presence of conc. H2SO4.This is based upon the tendency of Fe2+ to reduce nitrates to nitric oxide which reacts with Fe2+ to form a brown coloured complex.
The test is usually performed by adding dilute FeSO4 solution to an aqueous solution containing NO3¯ ion and then adding conc. H2SO4 slowly along the sides of the test tube. A brown ring at the interface between the solution and sulphuric acid indicates the presence of NO3¯ ion.
3Fe2+ +NO3¯ + 4 H+ —-> NO + 3Fe3+ + 2H2O
Fe3+ + NO + 5 H2O —-> [Fe (H2O)5 NO]2+
Pentaaquanitrosyl iron (II) ion
Uses of Nitric Acid
(i) It is used in the manufacture of ammonium nitrate for fertilizers.
(ii) It is used in the manufacture of sulphuric acid by lead chamber process.
(iii) It is used in the manufacture of explosives such as trinitro toluene (TNT), nitroglycerine, picric acid, etc.
(iv) It is used in the manufacture of dyes, perfumes and silk.
(v) It is used for the manufacture of nitrates for use in explosive and pyrotechnics.
(vi) It is used in picking of stainless steel and etching of metals.
(vii) It is also used as an oxidiser in rocket fuels.
(viii) It is used in the purification of gold and silver as aqua regia.
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
