NCERT Solutions, Question Answer and Mind Map for Class 12 Chemistry Chapter 8, “The d and f Block Elements,” is a study material package designed to help students understand the properties and characteristics of transition metals, their electronic configuration, and the trends in their chemical behaviour.
NCERT Solutions provide detailed explanations and answers to the questions presented in the chapter. The solutions cover all the topics in the chapter, including electronic configurations of transition elements, their oxidation states, magnetic properties, and complex formation reactions. They also provide tips on how to answer different types of questions, including short answer, long answer, and multiple-choice questions.

The question-answer section of the chapter covers a wide range of topics, from the properties of transition metals, such as their atomic and ionic radii, to the electronic configurations of f-block elements and their applications in everyday life. It also includes questions on the trends in the chemical behaviour of transition metals, including the effect of atomic and ionic sizes on their reactivity.
The mind map provides a visual representation of the key topics covered in the chapter, allowing students to understand the connections between different concepts and ideas. The mind map covers the electronic configurations of transition metals, their oxidation states, and the properties of f-block elements.
NCERT Solution / Notes Class 12 Chemistry Chapter 8 The D And F Block Elements With Mind Map PDF download
Introduction to d-block elements
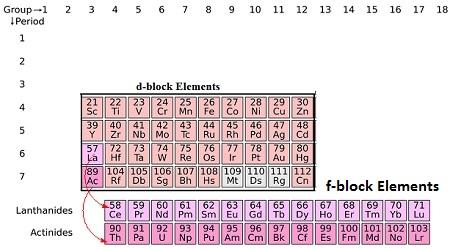
- In the periodic table the d block consist of the elements of group 3 to 12.
- The d orbital of the d-block elements in four periods are filled.
- The three series of the transitionmetals are 3d series from Sc to Zn, 4d series from Y to Cd and 5dseries from La
- to Hg.
- The fourth 6dseries begins from Ac and is incomplete till now.
Position of d-block in the periodic table
- The d-block elements are found in the middle section of s- and p- block elements in the periodic elements.
- This lead to its name ‘transition’ due to its position between s- and p- block elements.
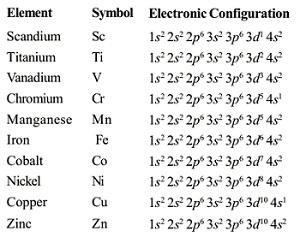
Electronic Configurations of the d-Block Elements
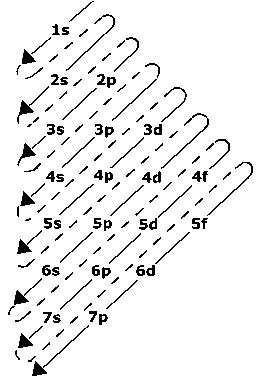
- The electronic configuration of d-block elements is(n-1) d1–10 ns1–2. They have two incomplete outershells.
- Where (n–1) = Inner d orbitals having electrons from 1-10.
- ns = Outermost orbital may have one or two
- (n-1) d10 n s2 represents the electronic configurations of Zn, Cd and Hg.
- They exhibit variable valency that differ by units of one.
Physical Properties
- The transition metals are hard and tough. They have low volatility butZn, Cd and Hg are an exception.

- They have high melting and boilingpoints due to the greaterquantity of electrons from (n-1) d along with the ns electrons metallic
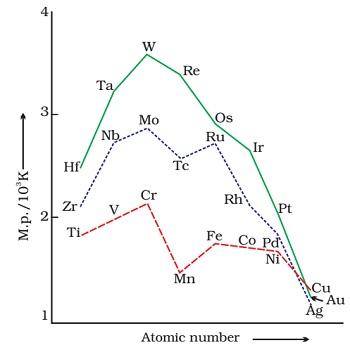
Fig. The trends in melting point of d-block elements.
- Metals possessing high boiling point are noble in their
- The metals belonging to second and third series have greater enthalpies of atomisation than the elements belonging to first series.
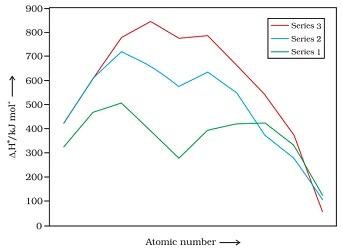
Fig. Enthalpies of atomisation
Metallic characteristics:
- All transition metals exhibit metallic character.

- They are good conductors of heat and electricity.

Fig. Metal is used at the tip of the plug that is inserted into the socket
- They are hard and tough.
- Being metal they exhibit the property of malleability, ductility and sonorousity.

Fig. Aluminium is beaten into thin sheets to make aluminium foil used to pack food (Malleability)

Fig. bells in temples are made of metal that when struck against hard surface produces sound (Sonorousity)
Fig. Metals are drawn into wires (Ductility)
- They form alloys by combining with some other metals.
- They are found to exist in face- centered cubic (fcc) structure, hexagonal close-packed (hcp) structure and body-centered cubic (bcc) structure.
- The transition elements exhibit covalent as well as metallic bonding within the atoms.
Atomic radii
- The atomic radii of the elements of 3d-series decreases as the atomic number increases.
- The atomic radii increase from 3d to 4d, the atomic radii of the 4d and 5d transition series are very close due to lanthanoid contraction. For example, Zirconium and Hafnium.
- This decrease in the metallic radius due to increase in the atomic mass leads to an increase in the density of elements. Consequently the density increases from titanium to copper.
Ionisation Enthalpies
- Transition elements have small size which results in high ionization energy.
- They exhibit less electro positivity than the s-block elements due to their ionization potentials lying between S and P block elements.
- They form covalent compounds.
- The d-block elements exhibit an increase in the ionization potentials from left to right due to the screening effect of the new electrons added into the (n-1) d subshell.
- The first transition series exhibit an increase in the second ionisation energies with the increase in atomic number due to stable electronic configuration.
- Ionization energy decreases down the group.
- Ionization energy increases across the period.
Oxidation States
- The number allotted to an element in a compound representing the number of electrons lost or gained by an atom of the element of the compound is called oxidation state.
For example, the electron configuration of copper is [Ar] 3d10 4s1. It attains noble gas configuration by losing one electron. The energy required to lose one more electron is very less and hence copper loses 2 electrons and forms Cu2+ ion. Therefore copper exhibits +1 and +2 oxidation state. But +2 oxidation states are more common.
It forms compounds like CuCl2 and also with oxygen like CuO. In both the cases the oxidation state of Cu is +2.
- Transition elements exhibit varying oxidation states due to the minor energy difference between ns and (n -1) d orbitals.
- Along with ns electrons, (n -1) d electrons takes part in bonding. But due to the availability of few electrons for bonding Scandium does not show variable oxidation states.
- Due to presence of more d electrons, zinc has less orbital available for bonding and hence does not exhibit varying oxidation state.
- Among d-block elements the elements belonging to 8th group exhibit maximum oxidation state.
- Among the elements of 3d –series Manganese belonging to 7th group exhibits maximum oxidation state.
- Among the elements of 4d-Series Ruthenium belonging to 8th group exhibits maximum oxidation state.
- Among the elements of 5d-Series Osmium belonging to 8th group exhibits maximum oxidation state.
- The oxidation number of a free element is always 0.
- Oxidation number of (group I) elements like Li, Na, K, Rb, Cs is +1.
- Oxidation number of (group II) elements like Be, Mg, Ca, Sr, Ba is +2.
- Oxidation number of oxygen is -2.
- For example, oxidation state of Phosphorous in the compound HPO32- can be calculated by the following method:
Oxidation state of H = +1
Oxidation state of O = -2
Oxidation state of O3 = 3(-2) [Since it has 3 atoms of oxygen.]
Overall oxidation state of the compound = -2
Let P represent the oxidation state of Phosphorous.
Therefore,
HPO32- = +1+P+3(-2) = -2
- P = +3
Standard hydrogen electrode
- The electrode is connected to a standard hydrogen electrode (SHE) to constitute a cell
- It consists of a platinum electrodecoated with a layer of platinum black.
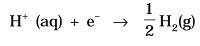
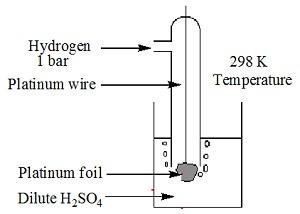
- The electrode is immersed in an acidicsolution and the pure hydrogen gas is bubbled through it.
- Theconcentration of the reduced form and the oxidized form of hydrogen issustained at unity with following conditions:
- Pressure of hydrogen gas = 1 bar
- Concentration of hydrogen ion in the solution = 1 molar
Ecell = Ecathode – Eanode
Ecell = Ecathode – 0 = Ecathode
- The measured Emf of the cell:
Pt| H2 (1 bar)| H+ (1M) || Cu2+ (1M)| Cu is 0.34 V.
The positive value of the standard electrode potential signifies the easy reduction of Cu2+ ions than H+ ions.
- The measured Emf of the cell
Pt| H2 (1 bar)| H+ (1M) || Zn2+ (1M)| Zn is -0.76 V.
The negative value of the standard electrode potential signifies that the hydrogen ions oxidizes the zinc (or it can be said that zinc can reduce hydrogen ions).
- An electrode with standard electrode potential greater than zero is stable in its reduced form compared tohydrogen gas.
- Whereas an electrode with negative standard electrode potential is less stable in its reduced form compared to hydrogen gas.
- This decreases the standard electrodepotential which in turn decreases the oxidizing power ofthe specific electrode on the left and increases the reducing power of the electrodeto the right of the reaction.
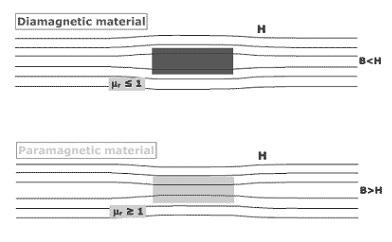
Magnetic Properties
Substances, depending on their behaviour in an external magnetic field, are classified into 2 types:
Paramagnetic
- They are weakly attracted on application of magnetic field due to presence of one or more unpaired electrons that gets attracted by the magnetic field.
- Application of a magnetic field magnetizes the paramagnetic substances in the same direction but lose their magnetism in the absence of magnetic field.
- O2, Cu2+, Fe3+, Cr3+ are some examples of such substances.
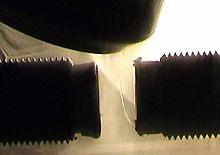
Fig. A trickle of liquid oxygen is deflected by a magnetic field, illustrating its paramagnetism.
Diamagnetic
- They are weakly repelled by a magnetic field due to the absence of unpaired electrons.
- They are weakly magnetized on application of magnetic field in opposite direction.
- Pairing of electrons cancels out their magnetic moments and they lose their magnetic character.
- For example, H2O, NaCl and C6H6 are some examples of such substances.

In 3d series the orbital angular momentum of the electrons of the elements is less due to which they exhibit less contribution.
The magnetic moment for these elements is calculated using the spin only formula
μ = √(n(n+2))
Where n = number of unpaired electrons
μ = magnetic moment in units of Bohr Magneton (BM).
PROBLEM: Calculate the ‘spin only’ magnetic moment of M2+(aq) ion (Z = 27).
SOLUTION: Z = 27 = [Ar] 3d7 4s2
M2+ = [Ar] 3d7

This means that it has 3 unpaired electrons.
n = 3
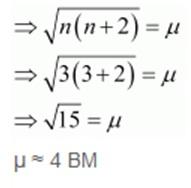
Formation of Coloured Ions
- An electron from a lower energy d orbital is excited to a higherenergy d orbital, the energy of excitation corresponds to the frequencyof light absorbed.
- This frequency generally lies in the visible
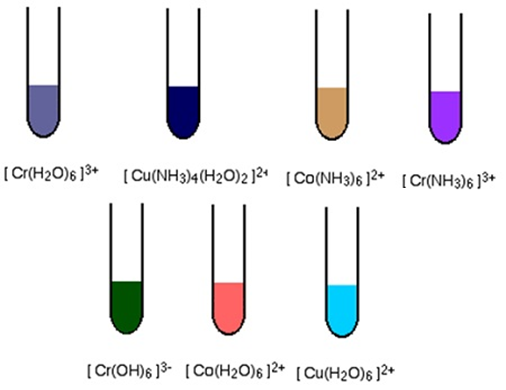
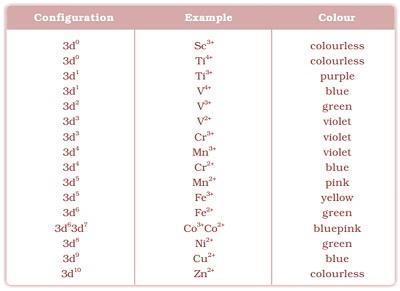
- The colour of the transition metal ions is due to the presence of unpaired or incomplete d-orbitals.
- The absorption of visible light and hence coloured nature of the transition metal cations is due to the promotion of one or more unpaired d-electron from a lower to a higher level within the same d-subshell. This promotion requires small amount of energy available in the visible light.
- Sc3+, Ti4+, Cu+ and Zn2+ have either entirely empty or entirely filled 3d-orbital, i.e. they do not have any unpaired d-electron, and hence appear colourless.
Formation of Complex Compounds
- The cations of transition metals have great tendency to form complexes with several molecules or ions called ligands.
- The bonds involved in the formation of complexes are coordinate and hence the complexes are called coordinate complexes.
- The structure of these complex ions is linear, square, planar, tetrahedral, octahedral depending upon nature of hybridization of metal ions.
- The weak ligand like CO, NO forms complexes only when transition metals are in zero due to the availability of vacant orbitals in the donor atom of the ligand in addition to lone pair.
- The highly electronegative and basic ligand like F-, Cl- can form complexes with transition metals even though there are in high oxidation states due to the presence of small, highly charged or neutral ligands with lone pair of electrons that can form strong sigma bond by donating a lone pair of electrons.
- In a transition series the stability of complexes increases with the rise in atomic number.
- The transition metal atom reveals multiple oxidation state; the higher valent ion forms more stable complexes.
- A few examples are:
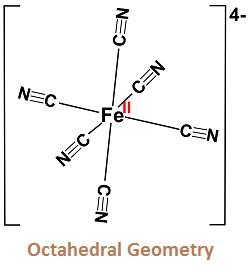
- [Fe (CN)6]3–
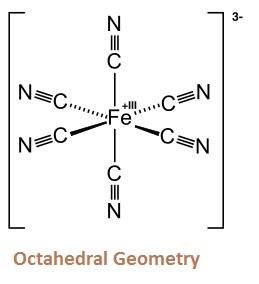
- [Fe(CN)6]4–
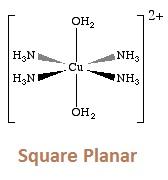
- [Cu(NH3)4]2+
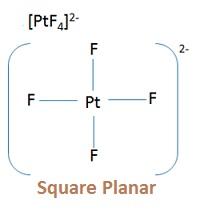
- [PtCl4]2–
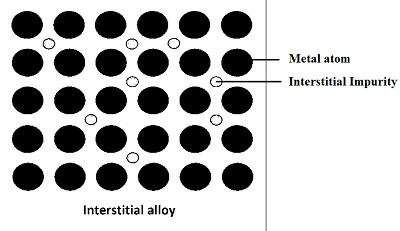
Formation of Interstitial Compounds
- Transition elements in combination with small atoms like H, B, C, N etc. leads to the formation of interstitial compounds that are non-stoichiometric in composition.
E.g.: TiH1.3, VH0.54
- The interstitial compounds so formed are chemically inert having higher melting points as compared to pure metals. These componds are hard and tough and keeps metallic conductivity.
Alloy Formation
- Alloys are homogeneous mixtures of more than one metal that can displace another metal from the crystal lattice due to their comparable sizes. This leads to the formation of alloys.
- The alloys so formed are hard with high melting points. For example, chromium, vanadium, tungsten, manganese, molybdenum are the ferrous alloys.
- Some other examples are brass (alloy of copper + zinc), stainless steel, bronze (alloy of copper + tin), etc.

Non-stoichiometric Compounds
- The compounds in which the there is no conformity in chemical composition with the ideal chemical formula are called non-stoichiometric compounds.
- These compounds are formed due to variable valency in transition metals and also due to the defects arising in solid state.
- The compounds formed with O, S, Se, Te, Fe, Zn etc. are examples of such compounds.
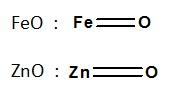
Preparation of K2Cr2O7
Potassium dichromate, (K2Cr2O7) is an orange-ish inorganic chemical reagent. In different laboratory or industry it is basically used as an oxidizing agent usually for alcohols.
It can be prepared through the following process:
- At first the fusion of chromite ore FeCr2O4 with sodium or potassium carbonate in the presence of access of air.
4FeCr2O4 + 8Na2CO3 + 7O2 –> 8Na2CrO4 + 2FeO3 + 8CO2
- Solution of sodium chromate is first filtered and then acidified with a solution of sulfuric acid which results in an orange sodium dichromate solution Na2Cr2O7 2H2O can be crystallized.
2Na2CrO4 + 2H+ –> Na2Cr2O7 + 2Na+ + H2O
- Sodium dichromate is more soluble than potassium dichromate and therefore it is fused with KCl that leads to the formation of orange crystals of potassium dichromate.
Na2Cr2O7 + 2KCl –> K2Cr2O7 + 2NaCl
- At pH equal to 4 the dichromates and chromates exists in equilibrium and can be inter convertible.
2CrO4 2- + 2H2+ –> Cr2O72- + H2O
Cr2O7 2- + 2OH- –> 2CrO42- + H2O
- The yellow colour of chromate changes to orange coloured dichromate in the presence of acidic medium whereas the dichromate changes into chromate in the presence of basic medium.
2CrO42- + 2H+ –> 2HCrO4– (Hydrogen chromate)
2HCrO4- –> Cr2O72- + H2O Dichromate (orange)
- The chromate ion is tetrahedral and the dichromate ion consists of two tetrahedral sharing at one corner, with Cr-O-Cr bond angle 126 degree.
Properties of Potassium dichromate (K2Cr2O7)
Oxidizing properties
Potassium dichromate is a powerful oxidizing-agent in an acidic medium.
Cr2O7 2- + 14H+ + 6 electron –> 2Cr3+ + 7H2O
It oxidizes iodides to iodine.
Cr2O7 2- + 14H+ + 6I– –> 2Cr 3+ + 7H2O + 3I2
It oxidizes ferrous salts to ferric salts.
Cr2O7 2- + 14H+ + 6 Fe2+ –> 2Cr 3++ 7H2O + 6Fe3+
It oxidizes stannous salts to stannic salts.
Cr2O7 2- + 14H+ + 3Sn 2+ –> 2Cr 3+ + 7H2O + 3Sn 4+
It oxidizes H2S to sulphur.
Cr2O7 2- + 8H+ + 3H2S –> 2Cr 3+ + 7H2O + 3S
Action of heat
Application of heat leads to the decomposition of Potassium dichromate leading to the formation of potassium chromate, chromic oxide and oxygen.
4K2Cr2O7 -Heat-> 4K2CrO4 + 2CrO3 + 3O2
Preparation of Pottassium Permanganate (KMnO4)
Pottassium Permanganate (KMnO4) is a dark purple solid consisting of two ions: a potassium ion (K⁺) and a permanganate ion (MnO− 4). It is a strong oxidizing agent and also possess medication properties due to which it is extensively used to clean wounds and in dermatitis.
- Fusion of powdered Pyrolusite ore (MnO2) with an alkali metal hydroxide like KOH in the presence of air or an oxidizing agent like KNO3 leads to the formation of dark green potassium Manganate (K2MnO4) which disproportionate either in a neutral or acidic medium and results in the formation of potassium permanganate.
2 MnO2 + 4 KOH + O2 –> 2K2MnO4 + 2H2O
3 MnO42- + 4H+ –> 2MnO4– + MnO2 + 2H2O
- Potassium permanganate is commercially prepared by an alkaline oxidative fusion of Pyrolusite ore (MnO2) and again by the electrolytic oxidation of manganate (4) ion.
2 MnO2 + 4KOH + O2 –> 2K2MnO4 + 2H2O
MnO42- + (electrolytic oxidation) –> MnO4– + e–
Introduction to f-Block Elements
- These elements are also called inner transition elements because the last electron enters (n−2) f- orbital, i.e. inner to the penultimate energy level and forms a transition series.
- The general electronic configuration of these elements can be given as
Hence, they have three incomplete shells, viz. (n−2), (n−1) and nth.
Classification of f-block elements
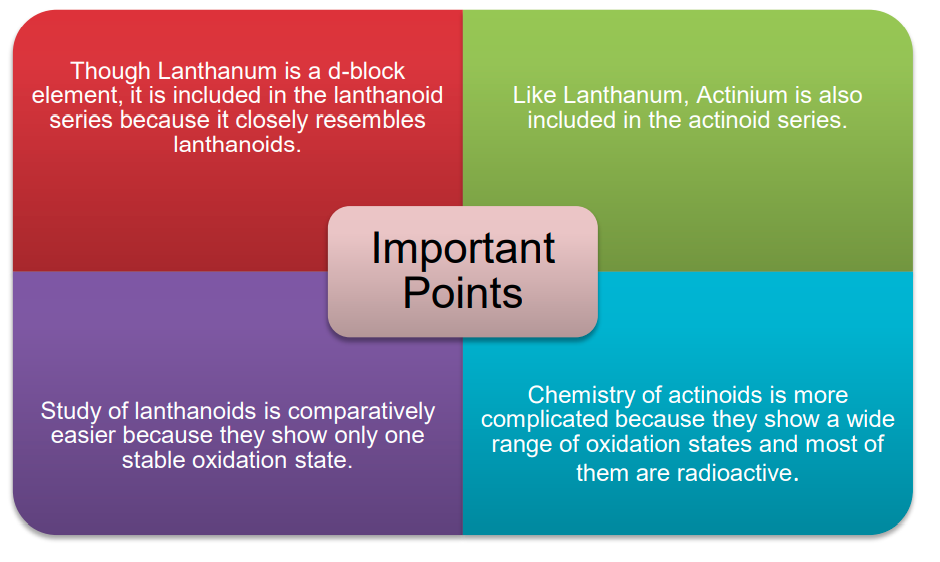
- Lanthanoids:
- They are called Lanthanoids because they come immediately after Lanthanum.
- They are also called 4f-block elements or first inner transition series elements or lanthanides or lanthanons.
- Actinoids:
- They are called Actinoids because they come immediately after Actinium.
- They are also called 5f-block elements or second inner transition series elements or actinides or actinons.
The Lanthanoids:
- Electronic Configuration:
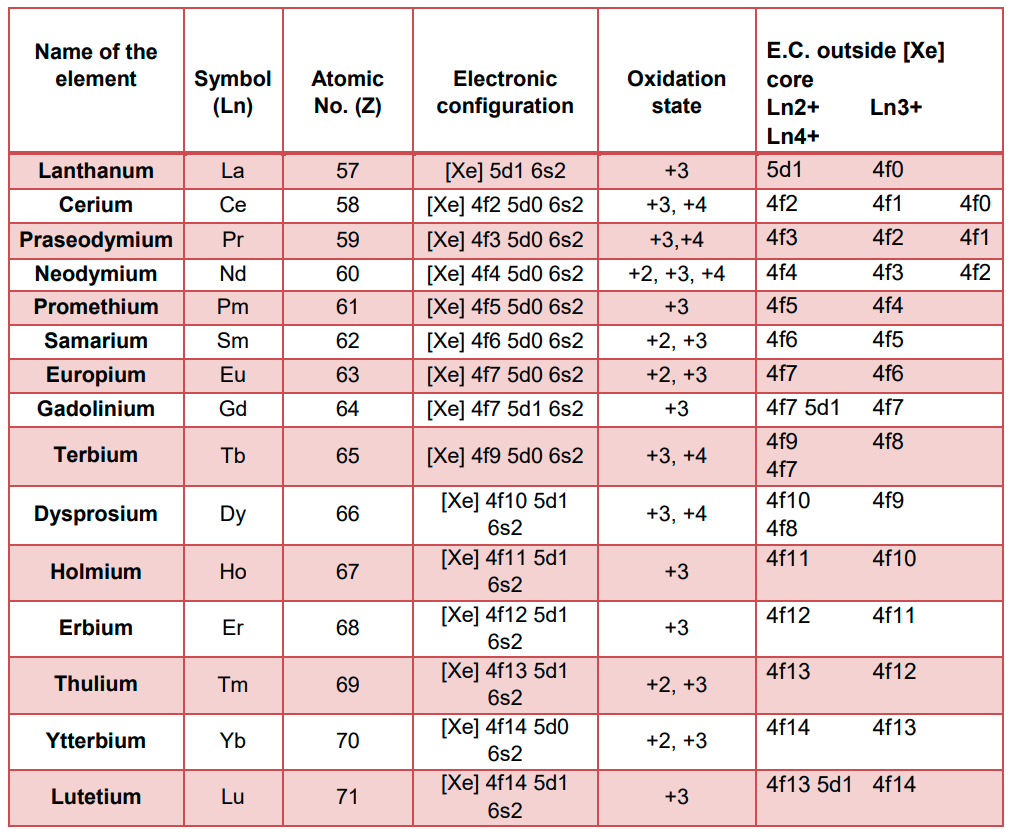
- Lanthanoids have the common electronic configuration of 6s2 and electrons occupying 4f level variably. The electronic configuration of all the tripositive ions are of the form 4fn (n = 1 −14) with increasing atomic number.
- The electronic configuration of Europium (Z = 63) is 4f7 6s2 and that of Gadolinium (Z = 64) is 4f7 5d1 6s2. This can be explained on the basis of extra stability of the half-filled orbitals in their cores.
- The electronic configuration of Ytterbium (Z = 70) is 4f14 6s2 and that of Lutetium (Z = 71) is 4f14 5d1 6s2. This is also explained on the basis of extra stability of the filled orbitals in their cores.
- Oxidation states:
- The common oxidation state of the lanthanoids is +3.
- The +2 and +4 oxidation states are very less common. These are exhibited by those elements which attain a stable electronic configuration of f0, f7 or f14 by losing 2 or 4 electrons.
- Here, each element tries to attain the stable oxidation state by losing or gaining electrons, i.e.
+3. Hence, Sm2+, Eu2+ and Yb2+ ions in solutions are good reducing agents and aqueous solutions of Ce4+ and Tb4+ are good oxidising agents.
- Atomic and ionic radii of Lanthanoids:
- In lanthanoids, if the atomic number increases, atomic and ionic radii decrease from La3+ to Lu3+.
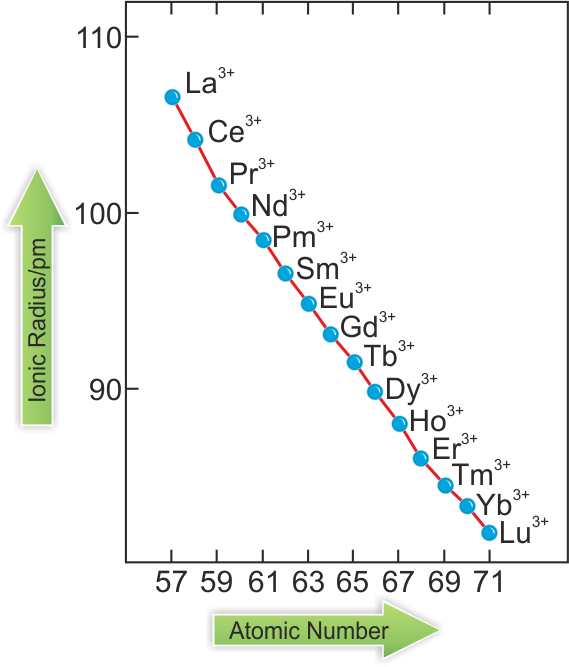
- Causes of lanthanoid contraction:
- When we move from left to right along the lanthanoid series, the nuclear charge increases by one unit at each neighbouring element. The new electron is added to the same subshell. So, the attractive force between the electron and nucleus increases, and hence, the size decreases.
- When a new electron is added to the f-subshell, the shielding effect of one electron by another is not perfect due to the shape of f-orbitals. Such a shielding cannot balance the effect of increased nuclear charge. Hence, contraction occurs.
- Consequences of lanthanoid contraction:
- Difficulty in separation of lanthanoids:
- Because of the slight difference in ionic radii of lanthanoids, their chemical properties are similar. This makes separation of lanthanoids more difficult.
- Also, due to the difference in the size of lanthanoids, properties such as solubility, complex ion formation and hydration show differences. This helps separate the individual lanthanoids by the ion exchange method.
- Similarity in the size of elements belonging to the same group of the second and third transition series:
- The size of elements belonging to the second transition series is always greater than that of the elements belonging to the same group of the first transition series. Also, the size of the atom of the third transition series, i.e. after lanthanum, is nearly the same as that of the atom of the element belonging to the same group of the second transition series.
- Similarity in size of the atoms of the elements belonging to the same group of the 2nd and 3rd transition series is due to the effect of lanthanoid contraction.
- Effect on the basic strength of hydroxides:
Because the size of lanthanoid ions decreases from La3+ to Lu3+, the covalent character of the hydroxides increases, and hence, the basic strength decreases. Therefore, La(OH)3 is more basic, while Lu(OH)3 is weakly basic.
- Characteristics of Lanthanoids:
- Silvery appearance and softness:
All lanthanoids are silvery white soft metals and tarnish easily in air. As the atomic number increases, their hardness also increases.
- Melting point:
They have a very high-melting point in the range 1000−1200 K except samarium, which has a high melting point of about 1623 K.
- Electrical and thermal conductivity:
They have metallic characteristics, and hence, they are good conductors of heat and electricity.
- Density:
They have high densities in the range of 6.77−9.74 gcm−3. Density and other properties differ smoothly with increasing atomic number except in Eu and Yb.
- Colour:
They are silvery white. Most of the trivalent ions are coloured in solid and in aqueous solution. This is due to f–f transition.
- Magnetic behaviour:
All the lanthanoids except La3+ and Lu3+ show paramagnetism. This property is due to the presence of unpaired electrons in the incomplete 4f subshell.
- Ionisation enthalpies:
The first ionisation enthalpies of lanthanoids are about 600 kJ mol−1 and the second is about 1200 kJ mol−1. The third ionisation enthalpy is low if it leads to a stable electronic configuration,
i.e. empty, half-filled or completely filled.
- Electropositive character:
They are highly electropositive because they possess low ionisation enthalpy.
- Standard electrode potential:
The value of their standard electrode potential, i.e. Eo for half-reaction, M3+ (aq) + 3e– → M(s), lies in the range −2.2 to −2.4 V. Europium is an exception because its Eo value is −2.0 V.
- Reducing agents:
They easily lose electrons; hence, they are good reducing agents.
- Complex formation:
Because of their large size and low charge density, they do not have much tendency to form complexes. This tendency of complex formation increases with increasing atomic number.
- Chemical behaviour:
The first few elements of the series are more reactive like calcium. As the atomic number increases, their behaviour becomes similar to that of aluminium. They show the following properties:
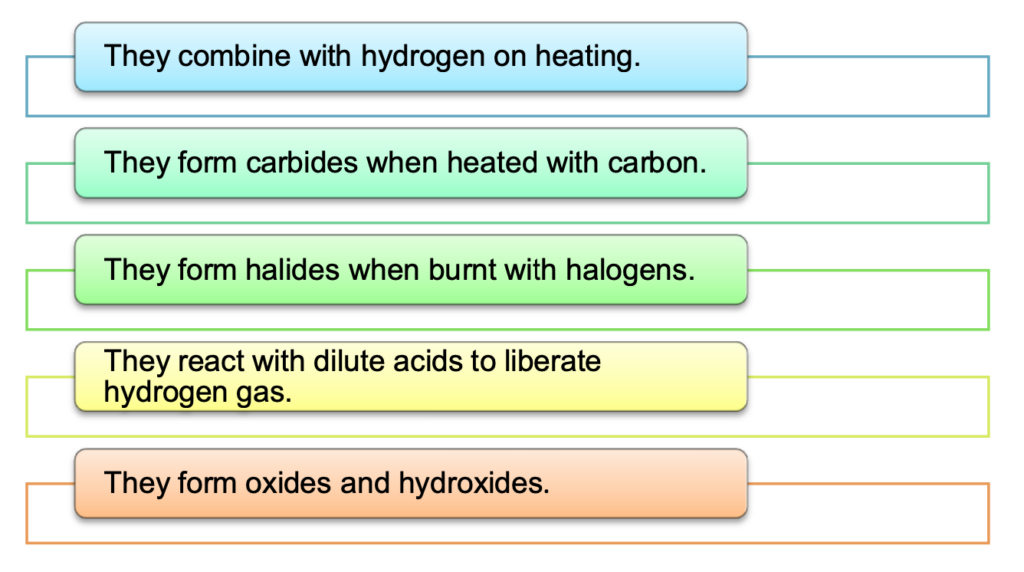
- Uses of lanthanoids:
- It is mainly used in the production of alloy steels to improve the strength of steel. A well-known alloy is mischmetal which has the following composition:
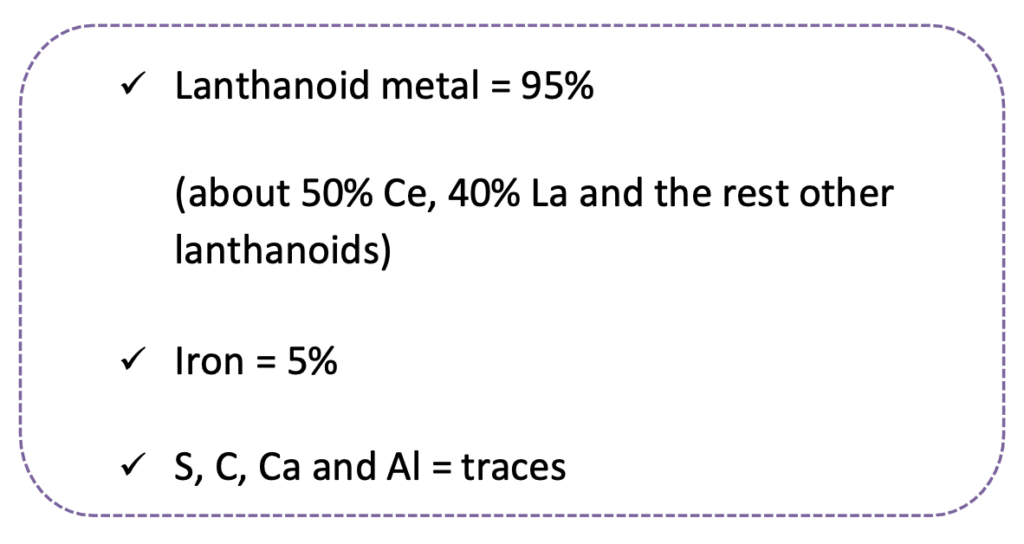
The mischmetal is mostly used in making a magnesium-based alloy. It is pyrophoric alloy, i.e. an alloy which emits sparks when struck. It is used in making bullets, shells and lighter flints.
- Their oxides are used in the glass industry—for polishing glass and to make optical glass.
- Mixed oxides of lanthanoids are used as catalysts in petroleum cracking.
- Ceric sulphate is a well-known oxidising agent used in volumetric analysis.
Actinoids
- Electronic Configuration:
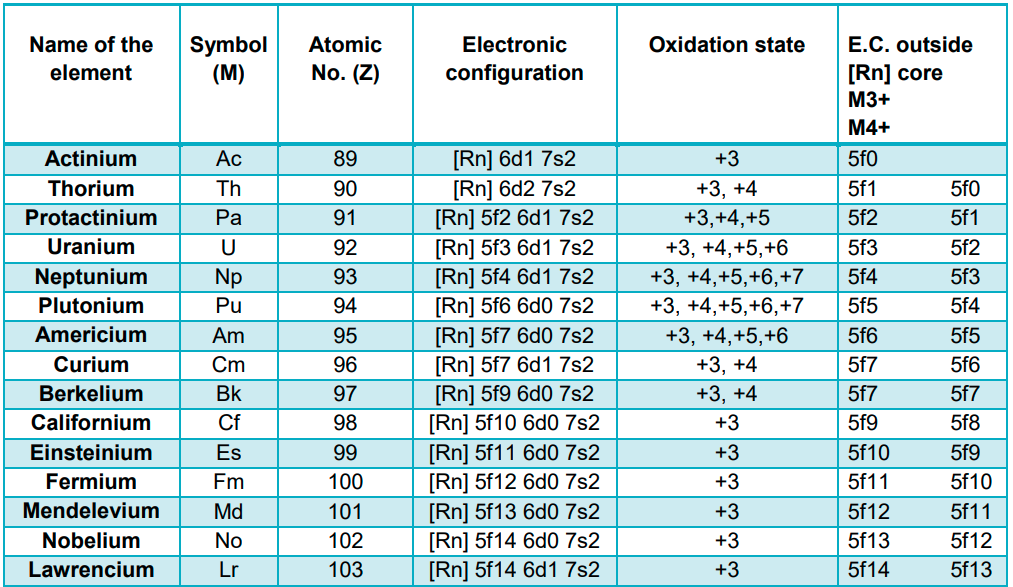
- All the actinoids have common 7s2 configuration, and filling of the 5f and 6d subshells is variable.
- The 14 electrons are being added to 5f, except in thorium (Z = 90), but this filling of the 5f subshell continues further after thorium till 5f orbitals are complete at Z = 103.
- Irregularities in the electronic configurations of actinoids are concerned with the stabilities of f0, f7 and f14 configurations.
- Although the 4f and 5f orbitals have similar shapes, 5f is less deeply buried than 4f. Hence, 5f electrons can participate in bonding.
- Oxidation state:
- The common oxidation state of all actinoids is +3.
- Actinoids also possess the oxidation state of +4. Some of them show higher oxidation state. The oxidation state gradually increases from the extreme left to the middle of the series and then decreases.
- The compounds of actinoids with +3 and +4 oxidation states undergo hydrolysis.
- Ionic radii and actinoid contraction:
- Like lanthanoids, actinoids also show contraction due to the poor shielding effect of the 5f- electrons.
- So, the radii of the atoms or ions of actinoids decrease gradually along the series.
- This contraction is greater from element to element in a series because 5f orbitals extend in the space beyond 6s and 6p orbitals.
- Characteristics of Actinoids:
- Silvery appearance:
Actinoids are metals with silvery appearance.
- Structural variability:
They have much more regularities in their metallic radii; hence, they show great structural variability.
- Colour:
They are silvery white metals. Their cations are generally coloured. The colour of these cations depends on the number of 5f-electrons.
The cations containing zero 5f electrons or seven 5f electrons are colourless. The cations containing 2−6 5f electrons are coloured.
This colour mainly arises because of f–f transition.
- Melting and boiling points:
Actinoids have high melting and boiling points. They do not show any gradual change even with increasing atomic number.
- Density:
With the exception of thorium and americium, all actinoids have high densities.
- Ionisation enthalpies:
They have low ionisation enthalpies than lanthanoids. This is because 5f is less penetrating than 4f and hence is more effective in shielding from nuclear charge.
- Electropositive character:
They are highly electropositive.
- Magnetic behaviour:
They are strongly paramagnetic. The change in magnetic susceptibility of actinoids with increasing number of unpaired electrons is the same as lanthanoids, but the values are higher for actinoids.
- Reducing agents:
All the actinoids are strong reducing agents.
- Radioactivity:
All the actinoids are radioactive. The first few members of the series have long half-lives. The remaining have half lives ranging from very few days to few minutes.
- Chemical behaviour:
They are highly reactive in the crushed form. They show the following properties:

- Uses of actinoids:

Comparison of Lanthanoids and Actinoids
Similarities:
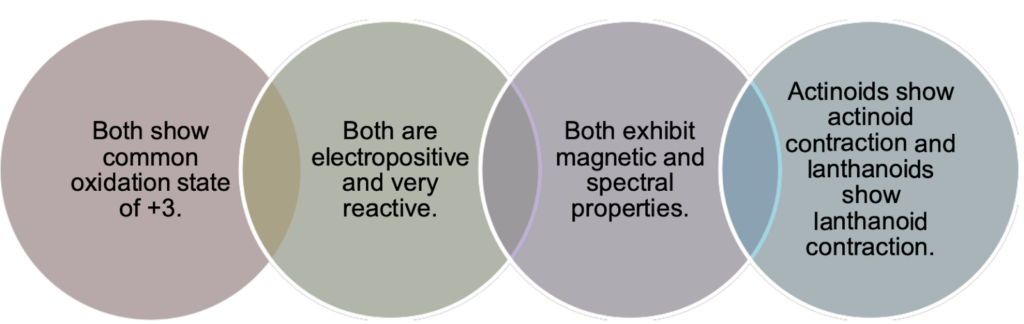
Differences:
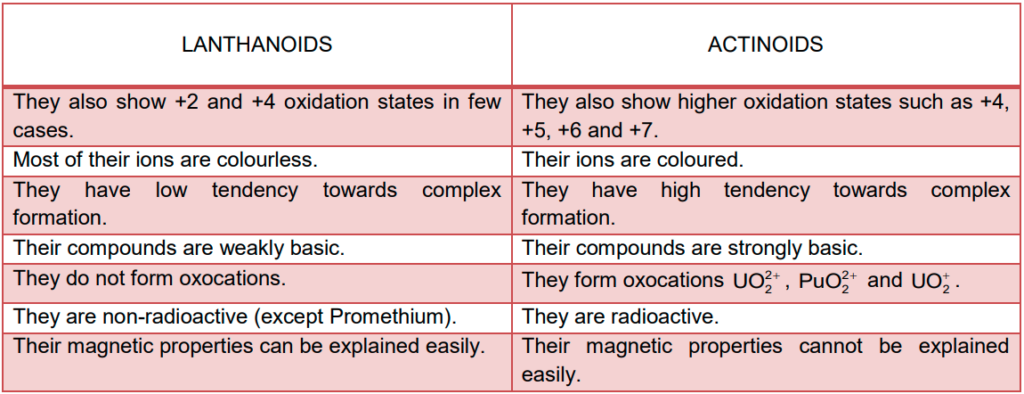
Comparison of Actinoids with Lanthanoids
General Characteristics and Comparison of Actinoids with Lanthanoids
(i) Electronic configuration
The general electronic configuration for actinoids is [Rn]86 5f1-14 6d0-1 7s2 and for lanthanoids is [Xe]54 4f0-14 5d0-1 6s2.
(ii) Atomic and lonic sizes
Like lanthanoids the ionic radii of actinoids gradually decrease across the series due to the poor screening effect of nuclear charge exerted by the f electrons.
(iii) Oxidation states
The lanthanoids exhibit +3 oxidation states. Some elements may exhibit +2 and +4 oxidation states due to extra stability of fully – filled and half – filled orbitals.
On the other hand Actinoids also exhibit +3 oxidation state. They also exhibit varying oxidation states due to the comparable energies of 5f, 6d, and 7s.
(iv) Chemical reactivity
- Earlier members of the lanthanide series are more reactive and are comparable to CSolution: They resemble Al with increasing atomic number.
- Finely divided Actinoids are highly reactive metals and when added to boiling water gives a mixture of oxide and hydride.
- At moderate temperatures Actinoids combine with most of the non-metallic elements.
- Actinoids remain unaffected by the action of alkalies but gets slightly affected by nitric acid due to the formation of a protective oxide layer.
Applications of d- and f-Block elements
Iron and steels are used for making tools, utensils, vehicles, bridges and much more.

TiO for the pigment industry and MnO2 for use in dry battery cells.

Zn and Ni/Cd is also used for battery industry.
Elements of Group 11 called the coinage metals.

V2O5 catalyses the oxidation of SO2 in the manufacture ofsulphuric acid.
TiCl4 with A1(CH3)3 forms the basis of the Ziegler catalystsused to manufacture polyethylene (polythene).
Iron catalysts are used inthe Haber process for the production of ammonia from N2/H2 mixtures.
Nickel catalysts enable the hydrogenation of fats
Wackerprocess the oxidation of ethyne to ethanal is catalysed by PdCl2.
Nickel useful in the polymerisation of alkynes and other organiccompounds such as benzene.
The photographic industry relies on thespecial light-sensitive properties of AgBr.
Problem: Write down the electronic configuration of:
(i) Cr3+ (iii)Cu+ (v)Co2+ (vii)Mn2+
(ii) Pm3+ (iv)Ce4+ (vi) Lu2+ (viii)Th4+
Solution:
(i) Cr3+: 1s2 2s2 2p6 3s2 3p6 3d3
Or, [Ar]183d3
(ii) Pm3+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f4
Or, [Xe]54 3d3
(iii) Cu+: 1s2 2s2 2p6 3s2 3p6 3d10
Or, [Ar]18 3d10
(iv) Ce4+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6
Or, [Xe]54
(v) Co2+: 1s2 2s2 2p6 3s2 3p6 3d7
Or, [Ar]183d7
(vi) Lu2+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d1
Or, [Xe]542f143d3
(vii) Mn2+: 1s2 2s2 2p6 3s2 3p6 3d5
Or, [Ar]18 3d5
NCERT Solutions for Class 12 Chemistry chapter wise
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
